Z-VAD-FMK: Mechanism, Applications, and Future Directions in Caspase Inhibition Research
This article provides a comprehensive analysis of Z-VAD-FMK, a pan-caspase inhibitor that has become an indispensable tool in cell death research.
Z-VAD-FMK: Mechanism, Applications, and Future Directions in Caspase Inhibition Research
Abstract
This article provides a comprehensive analysis of Z-VAD-FMK, a pan-caspase inhibitor that has become an indispensable tool in cell death research. It explores the foundational mechanism by which Z-VAD-FMK irreversibly binds to the catalytic site of caspases, its broad methodological applications in diverse disease models from endotoxic shock to noise-induced hearing loss, key challenges and optimization strategies for its use in complex biological systems, and its validation against other caspase inhibitors and therapeutic agents. Tailored for researchers, scientists, and drug development professionals, this review synthesizes current knowledge to guide experimental design and discusses the translational potential of caspase inhibition.
Unraveling the Core Mechanism: How Z-VAD-FMK Functions as a Pan-Caspase Inhibitor
Caspases are an evolutionarily conserved family of cysteine-dependent aspartate-specific proteases that serve as central regulators of programmed cell death (PCD), inflammation, and cellular homeostasis [1] [2]. These enzymes are synthesized as inactive zymogens (procaspases) and undergo proteolytic activation at specific aspartic acid residues, leading to the formation of active enzymes composed of large and small subunits [3]. The caspase family plays indispensable roles in maintaining organismal health by eliminating damaged, infected, or superfluous cells through tightly regulated cell death pathways [1]. Dysregulation of caspase-mediated processes is implicated in a wide spectrum of human diseases, including cancer, neurodegenerative disorders, autoimmune conditions, and inflammatory diseases, establishing them as prominent therapeutic targets for drug development [1] [3] [4].
The historical classification of caspases as either apoptotic or inflammatory has been reconsidered in light of emerging evidence demonstrating their multifunctional roles across various cell death pathways [2] [5]. Contemporary understanding recognizes that caspases form a complex regulatory network with significant crosstalk between different PCD pathways, leading to more inclusive classification systems based on structural domains, substrate specificity, and biological functions [2] [5]. This application note provides a comprehensive overview of caspase biology, their roles in cell death and disease, and detailed experimental protocols for studying caspase function, with particular emphasis on the pan-caspase inhibitor zVAD-FMK and its research applications.
Caspase Classification and Molecular Organization
Structural Classification Based on Pro-Domains
Caspases are primarily classified based on their N-terminal pro-domain structures and lengths, which dictate their activation mechanisms and functional specializations [2] [5]:
CARD-domain containing caspases: This group includes caspase-1, -2, -4, -5, -9, -11, and -12. The Caspase Activation and Recruitment Domain (CARD) facilitates protein-protein interactions through homotypic binding, enabling recruitment to specific signaling complexes such as inflammasomes and apoptosomes [1] [2].
DED-domain containing caspases: Caspase-8 and -10 contain two Death Effector Domains (DED) in their pro-domains. These domains mediate interactions with adapter proteins like FADD in the Death-Inducing Signaling Complex (DISC), initiating extrinsic apoptosis [1] [6].
Short or no pro-domain caspases: Executioner caspases including caspase-3, -6, and -7 possess short pro-domains and are typically activated downstream by initiator caspases through proteolytic cleavage [3] [2].
Table 1: Caspase Classification Based on Pro-Domains and Primary Functions
| Structural Group | Caspase Members | Activation Complex | Primary Functions |
|---|---|---|---|
| CARD-domain | Caspase-1, -4, -5, -11 | Inflammasome | Pyroptosis, cytokine maturation (IL-1β, IL-18) |
| CARD-domain | Caspase-2, -9, -12 | PIDDosome, Apoptosome | Intrinsic apoptosis, ER stress-induced apoptosis |
| DED-domain | Caspase-8, -10 | DISC, RIPK1-FADD-caspase-8 complex | Extrinsic apoptosis, necroptosis regulation |
| Short/No Pro-domain | Caspase-3, -6, -7 | Activated by initiator caspases | Execution of apoptosis, substrate cleavage |
Traditional Functional Classification
Despite the limitations of traditional categorization, understanding the historical classification provides context for caspase functions [2] [5]:
Inflammatory Caspases: Caspase-1, -4, -5, and -11 (murine homolog of human caspase-4/5) primarily regulate inflammatory responses through proteolytic activation of cytokines and induction of pyroptotic cell death [3] [2].
Apoptotic Initiator Caspases: Caspase-2, -8, -9, and -10 initiate apoptotic signaling through either extrinsic (death receptor) or intrinsic (mitochondrial) pathways [3] [6].
Apoptotic Executioner Caspases: Caspase-3, -6, and -7 serve as the primary effectors of apoptosis, cleaving numerous cellular substrates to orchestrate cellular dismantling [3] [2].
Caspase Functions in Programmed Cell Death Pathways
Apoptosis
Apoptosis represents a non-lytic, generally non-inflammatory form of PCD essential for development, tissue homeostasis, and elimination of damaged cells [1] [2]. This process occurs through two main pathways:
Extrinsic Pathway: Initiated by extracellular death ligands (e.g., FASL, TRAIL) binding to death receptors, leading to DISC formation, caspase-8 activation, and subsequent direct activation of executioner caspases-3 and -7 [1] [6].
Intrinsic Pathway: Triggered by intracellular stress signals (e.g., DNA damage, oxidative stress) causing mitochondrial outer membrane permeabilization, cytochrome c release, apoptosome formation with caspase-9, and activation of executioner caspases [1] [2].
The following diagram illustrates the key caspases involved in extrinsic and intrinsic apoptosis pathways:
Pyroptosis
Pyroptosis represents a lytic, inflammatory form of cell death primarily executed by gasdermin family proteins [1] [2]. Key caspase-mediated pathways include:
- Canonical Pyroptosis: Inflammasome-activated caspase-1 cleaves GSDMD and pro-inflammatory cytokines IL-1β and IL-18 [1] [2].
- Non-canonical Pyroptosis: Caspase-4, -5, and -11 directly cleave GSDMD to induce pore formation and membrane rupture [1] [2].
- Caspase-8-mediated Pyroptosis: In certain contexts, caspase-8 can cleave GSDMC and GSDMD to initiate pyroptosis [1] [6].
- Caspase-3-mediated Pyroptosis: Activated caspase-3 cleaves GSDME to induce pyroptotic cell death when GSDME is expressed [2].
Regulation of Necroptosis and PANoptosis
Caspases play crucial regulatory roles in other cell death pathways:
Necroptosis: Caspase-8 serves as a critical negative regulator of necroptosis by cleaving key necroptotic components RIPK1 and RIPK3. Pharmacological inhibition of caspase-8 with zVAD-FMK or genetic ablation promotes RIPK1-RIPK3-MLKL-mediated necroptosis [1] [7].
PANoptosis: This recently described inflammatory cell death pathway integrates components from pyroptosis, apoptosis, and necroptosis. Multiple caspases, including caspase-1, -3, -7, and -8, are activated within PANoptosomes in response to specific stimuli [2] [6].
The following diagram illustrates caspase involvement across different cell death pathways:
Caspases in Disease Pathogenesis and Therapeutic Targeting
Disease Associations
Dysregulated caspase activity contributes to numerous human diseases:
Cancer: Defective apoptotic caspase signaling promotes tumor survival and progression, while inflammatory caspases can create a tumor-promoting microenvironment [1] [8].
Neurodegenerative Disorders: Excessive caspase activation contributes to neuronal loss in Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis [3] [4].
Autoimmune and Inflammatory Diseases: Aberrant inflammasome activation and caspase-mediated cytokine maturation drive pathology in rheumatoid arthritis, systemic lupus erythematosus, and inflammatory bowel disease [4].
Infectious Diseases: Pathogen sensing activates caspase-mediated cell death pathways as a host defense mechanism, but excessive activation can cause tissue damage [2].
Caspase-Targeted Therapeutic Approaches
Several strategies have been developed to target caspases for therapeutic purposes:
Small Molecule Inhibitors: Peptide-based and non-peptide caspase inhibitors have been developed, including broad-spectrum inhibitors (zVAD-FMK, Q-VD-OPh) and selective inhibitors (Ac-YVAD-CHO for caspase-1) [3].
Biological Agents: Monoclonal antibodies targeting specific caspases or their activation complexes represent emerging therapeutic approaches [4].
Gene Therapy: Approaches modulating caspase expression levels are under investigation for specific applications [4].
Table 2: Selected Caspase Inhibitors and Their Research Applications
| Inhibitor | Target Specificity | Research Applications | Key Characteristics |
|---|---|---|---|
| zVAD-FMK | Pan-caspase inhibitor (caspase-1, -3, -8, etc.) | Apoptosis and necroptosis studies; endotoxic shock models | Cell-permeable, irreversible inhibitor; promotes necroptosis at high concentrations [7] [3] |
| Q-VD-OPh | Broad-spectrum (caspase-1, -2, -3, -6, -8, -9) | Neurodegeneration, ischemia-reperfusion injury | Reduced toxicity compared to zVAD; potent apoptosis inhibitor [3] |
| VX-765 (Belnacasan) | Caspase-1 selective | Inflammatory disease models (arthritis, epilepsy) | Orally bioavailable; advanced clinical trials [3] |
| Ac-DEVD-CHO | Caspase-3 selective | Apoptosis mechanism studies | Reversible inhibitor; based on PARP cleavage sequence [3] |
| Emricasan (IDN-6556) | Caspase-3, -7, -8, -9 | Liver disease, ischemia-reperfusion injury | Orally active; pan-caspase inhibitor profile [3] |
Experimental Protocol: Assessing zVAD-FMK Effects in Endotoxic Shock Models
Background and Principle
The pan-caspase inhibitor zVAD-FMK (carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone) has demonstrated protective effects in murine models of LPS-induced endotoxic shock [7]. This protocol outlines the methodology for evaluating zVAD-FMK-mediated protection against endotoxic shock, focusing on survival outcomes, cytokine production, and immune cell responses.
Materials and Reagents
Table 3: Essential Research Reagents for Endotoxic Shock Studies
| Reagent | Function/Application | Example Specifications |
|---|---|---|
| zVAD-FMK | Pan-caspase inhibition | Dissolve in DMSO; working concentrations 5-20 μg/g body weight in vivo; 20-80 μM in vitro [7] |
| Lipopolysaccharide (LPS) | Endotoxic shock induction | Escherichia coli serotypes; 10-50 μg/g body weight for mortality studies [7] |
| C57BL/6 mice | Animal model | Female, 6-8 weeks old; appropriate ethical approvals required [7] |
| Cell culture media | Macrophage maintenance | DMEM supplemented with GM-CSF (10 ng/mL) for BMDM differentiation [7] |
| ELISA kits | Cytokine quantification | TNF-α, IL-6, IL-1β detection in serum and supernatants [7] |
| Flow cytometry antibodies | Immune cell profiling | CD11b, Gr-1, Ly6G, Ly6C for MDSC identification [7] |
Procedure
In Vivo Endotoxic Shock Model
- Animal Preparation: House C57BL/6 mice (6-8 weeks old) under specific pathogen-free conditions with approved ethical oversight.
- Experimental Groups: Randomly assign mice to following groups (n=8-10/group):
- Control (vehicle only)
- LPS only
- zVAD-FMK only
- LPS + zVAD-FMK (pre-treatment)
- LPS + zVAD-FMK (post-treatment)
- Drug Administration:
- Sample Collection:
- Collect serum samples at 6 hours post-LPS challenge for cytokine analysis.
- Harvest organs (liver, lung, spleen) at 12-24 hours for histopathological examination.
- Collect peritoneal cells at 6 and 12 hours for cell death and population analysis.
- Survival Monitoring: Monitor survival every hour for the first 24 hours, then every 6 hours for up to 96 hours post-LPS challenge.
In Vitro Macrophage Studies
- Bone Marrow-Derived Macrophage (BMDM) Generation:
- Flush bone marrow cells from mouse femurs and tibias with PBS.
- Culture cells in complete DMEM supplemented with GM-CSF (10 ng/mL) for 7 days, refreshing medium on day 3 [7].
- Experimental Treatments:
- Pre-treat BMDMs with zVAD-FMK (0, 20, 40, 80 μM) for 30 minutes.
- Stimulate with LPS (100 ng/mL) for designated timepoints.
- Assessment Endpoints:
- Cell Viability: Measure using CCK-8 assay after 48 hours of treatment.
- Cytokine Secretion: Collect culture supernatants for ELISA quantification of TNF-α, IL-6, and other inflammatory mediators.
- Cell Death Analysis: Assess necroptosis and apoptosis by flow cytometry using propidium iodide and Annexin V staining.
Data Analysis and Interpretation
Key parameters to assess zVAD-FMK efficacy in endotoxic shock:
- Survival Analysis: Generate Kaplan-Meier survival curves and compare using log-rank test.
- Cytokine Levels: Determine statistical significance of cytokine reduction in zVAD-FMK treated groups versus LPS-only controls.
- Histopathological Scoring: Evaluate tissue damage in liver and lung sections using standardized scoring systems.
- Immune Cell Populations: Quantify changes in macrophage and MDSC populations by flow cytometry.
The expected results based on published findings include significantly improved survival, reduced pro-inflammatory cytokine levels, decreased peritoneal macrophage numbers, and increased accumulation of myeloid-derived suppressor cells (MDSCs) in zVAD-FMK treated animals [7].
The following diagram illustrates the experimental workflow and key mechanisms of zVAD-FMK action in endotoxic shock:
The Scientist's Toolkit: Essential Research Reagents
Table 4: Key Research Reagents for Caspase Studies
| Reagent Category | Specific Examples | Research Applications |
|---|---|---|
| Caspase Inhibitors | zVAD-FMK, Q-VD-OPh, Ac-YVAD-CHO, Ac-DEVD-CHO | Determining caspase-specific functions; therapeutic potential assessment [7] [3] |
| Activity Assays | Fluorogenic substrates (DEVD-AFC, WEHD-AFC), Caspase-Glo assays | Quantifying caspase activation kinetics; screening inhibitor efficacy |
| Antibodies | Active caspase-3, cleaved caspase-8, GSDMD-NT, PARP cleavage | Detecting caspase activation and downstream signaling by western blot, IHC, flow cytometry |
| Cell Death Inducers | Staurosporine, TNF-α + Cycloheximide, Nigericin, LPS | Activating specific cell death pathways for mechanistic studies |
| Animal Models | Caspase knockout mice, RIPK3-/- mice, GSDMD-/- mice | Determining physiological functions of specific caspases in disease contexts |
Caspases represent central regulators of cell death and inflammation with profound implications for human health and disease. The complex interplay between different caspase family members across multiple cell death pathways highlights the need for sophisticated experimental approaches when investigating their functions. The pan-caspase inhibitor zVAD-FMK serves as a valuable research tool for deciphering caspase-mediated processes, with demonstrated efficacy in inflammatory disease models such as endotoxic shock. As our understanding of caspase biology continues to evolve, particularly with the emerging concepts of PANoptosis and non-apoptotic caspase functions, new therapeutic opportunities will undoubtedly emerge for targeting these critical enzymes in various disease contexts.
Caspases are an evolutionary conserved family of cysteine-dependent proteases that play essential roles in modulating critical biological processes, including programmed cell death (apoptosis) and inflammation [9]. These enzymes synthesize as catalytically inactive zymogens and require proteolytic activation to become functional enzymes that cleave their substrates at specific aspartic acid residues. The dysregulation of caspase-mediated apoptosis and inflammation contributes to the pathogenesis of various diseases, such as inflammatory diseases, neurological disorders, metabolic diseases, and cancer, making caspases attractive therapeutic targets [9]. Consequently, developing specific caspase inhibitors has become a major focus in biomedical research and drug development.
Among the numerous caspase inhibitors developed to date, Z-VAD-FMK (benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone) stands as a cornerstone tool compound. This pan-caspase inhibitor possesses broad reactivity against multiple caspase family members and has become indispensable for fundamental research into apoptosis and caspase biology [10] [11]. Originally designed for potential therapeutic applications, its utility as a drug was limited due to unforeseen cytotoxicity of a metabolic derivative [10]. However, as a research tool for investigating caspase-dependent processes, Z-VAD-FMK has proven exceptionally valuable, enabling scientists to dissect apoptotic signaling pathways and distinguish between caspase-dependent and independent cell death modalities [12].
Detailed Mechanism of Irreversible Binding
Structural Basis of Caspase Inhibition
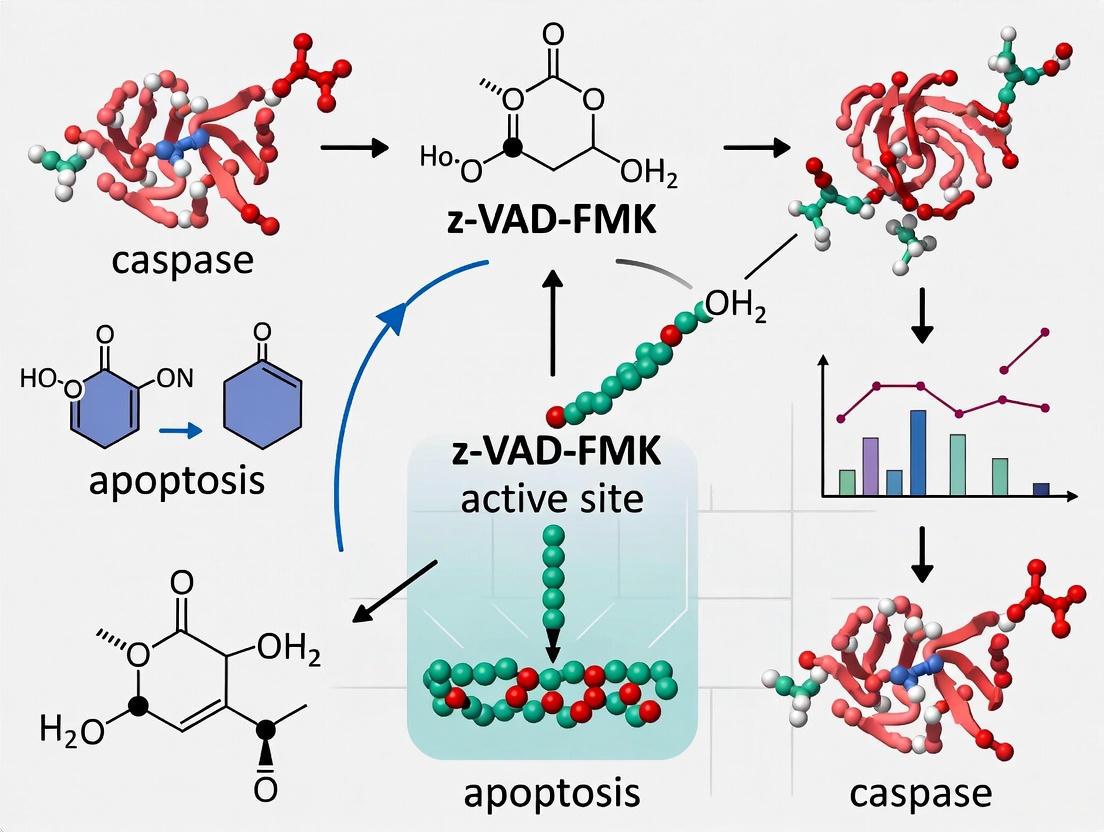
Z-VAD-FMK functions as an irreversible, broad-spectrum caspase inhibitor that covalently modifies the active site of caspase enzymes. Its molecular structure consists of three key components: a benzyloxycarbonyl (Z) group that enhances cell permeability, a Val-Ala-Asp (VAD) tripeptide sequence that mimics the natural substrate recognition motif of caspases, and a fluoromethyl ketone (FMK) warhead that mediates irreversible binding to the catalytic cysteine residue [12]. This strategic design enables the inhibitor to effectively penetrate cells and specifically target multiple caspase family members.
The inhibition mechanism proceeds through a covalent modification of the active site cysteine thiol group. The FMK group reacts with the nucleophilic cysteine residue in the caspase active site, forming a thioether bond that permanently inactivates the enzyme [12]. This interaction critically blocks the activation of pro-caspase CPP32 (caspase-3), thereby preventing its maturation from the zymogen state to the active enzyme [12]. Importantly, Z-VAD-FMK primarily targets the inactive zymogen forms of caspases rather than inhibiting the proteolytic activity of already-activated enzymes [12]. This specificity distinguishes it from competitive inhibitors that target active enzymes and makes it particularly valuable for preventing the initiation of the caspase cascade.
Structural Validation and Kinetics
Biophysical and structural studies have provided detailed insights into how Z-VAD-FMK and related inhibitors interact with caspase enzymes. Kinetic characterization reveals that Z-VAD-FMK inhibits caspase-3 and caspase-8 via a three-step kinetic mechanism [13]. For caspase-3 inhibition, this process involves two rapid equilibrium steps followed by a relatively fast inactivation step, while caspase-8 inhibition follows a distinct pathway with a rapid equilibrium step, a slow-binding reversible step, and an extremely slow inactivation step [13]. These kinetic differences highlight the variable inhibition profiles across different caspase family members.
Crystal structures of caspase-inhibitor complexes validate the rational design of peptidomimetic inhibitors like Z-VAD-FMK, illustrating in atomic detail how these compounds mimic natural peptide substrates [13]. The structural data confirm that the inhibitors occupy the substrate-binding cleft of caspase enzymes, with the aspartic acid residue in the VAD sequence engaging the S1 specificity pocket that normally recognizes aspartic acid in native substrates [13]. One caspase-8 structure also revealed binding at a secondary, allosteric site, suggesting a potential additional regulatory mechanism and providing a possible route for developing non-covalent small molecule modulators of caspase activity [13].
The following diagram illustrates the irreversible binding mechanism of Z-VAD-FMK to the catalytic cysteine of caspases:
Quantitative Characterization of Inhibition
Kinetic Parameters of Caspase Inhibition
The inhibition of different caspases by Z-VAD-FMK follows distinct kinetic pathways, as demonstrated by stopped-flow fluorescence assays that enable determination of individual kinetic parameters [13]. The table below summarizes the key kinetic characteristics for caspase-3 and caspase-8 inhibition:
Table 1: Kinetic parameters of Z-VAD-FMK-mediated caspase inhibition
| Caspase | Inhibition Mechanism | Key Steps | Inactivation Rate |
|---|---|---|---|
| Caspase-3 | Three-step mechanism | Two rapid equilibrium steps followed by inactivation | Relatively fast |
| Caspase-8 | Three-step mechanism | Rapid equilibrium, slow-binding reversible, then inactivation | Extremely slow |
| Pan-Caspase | Irreversible covalent binding | FMK warhead reaction with catalytic cysteine | Variable across caspase family |
The differential inhibition kinetics observed between caspase-3 and caspase-8 highlight the importance of considering caspase-specific effects when interpreting results from experiments using Z-VAD-FMK. While classified as a pan-caspase inhibitor, its efficiency and mechanism vary among different caspase family members, potentially influencing experimental outcomes in complex biological systems.
Practical Usage Parameters
For experimental applications, Z-VAD-FMK requires specific handling and usage conditions to maintain its activity and achieve effective caspase inhibition. The following table outlines the key practical parameters for working with this inhibitor:
Table 2: Experimental usage parameters for Z-VAD-FMK
| Parameter | Specification | Notes |
|---|---|---|
| Solubility | Readily soluble in DMSO (≥23.37 mg/mL) [12] | Insoluble in water or ethanol |
| Working Concentration | 10-100 μM [12] | Cell type-dependent optimization required |
| Treatment Duration | Pre-treatment 30-60 minutes before apoptosis induction [12] | |
| Storage Conditions | -20°C, desiccated [14] | DMSO stocks stable for months at -20°C |
| Cellular Models | THP-1, Jurkat, BMDMs, peritoneal macrophages [7] [12] | Effective in human and rodent cells |
Experimental Protocols
Protocol 1: Inhibition of Apoptosis in Cell Cultures
This protocol details the use of Z-VAD-FMK to inhibit caspase-dependent apoptosis in mammalian cell lines, such as THP-1 or Jurkat cells [12].
Materials:
- Z-VAD-FMK (lyophilized powder, >98% purity)
- Anhydrous DMSO (cell culture grade)
- Appropriate cell culture medium (e.g., RPMI-1640 for suspension cells)
- Apoptosis inducer (e.g., camptothecin, staurosporine)
- Cell viability assay reagents (e.g., CCK-8, MTT)
Procedure:
- Prepare inhibitor stock: Dissolve Z-VAD-FMK in anhydrous DMSO to prepare a 20 mM stock solution. Vortex thoroughly and aliquot to avoid repeated freeze-thaw cycles. Store at -20°C.
- Cell plating: Plate cells at appropriate density (e.g., 1×10⁵ cells/well in 96-well plates) in complete culture medium.
- Inhibitor pre-treatment: Add Z-VAD-FMK from stock solution to achieve final working concentrations (typically 20-80 μM). Include DMSO-only vehicle controls.
- Incubation: Pre-incubate cells with inhibitor for 30-60 minutes at 37°C, 5% CO₂.
- Apoptosis induction: Add apoptosis inducer (e.g., 10 μM camptothecin) and incubate for desired duration (typically 4-24 hours).
- Assessment: Analyze apoptosis inhibition using:
Protocol 2: In Vivo Application in Endotoxic Shock Models
This protocol describes the use of Z-VAD-FMK in murine models of endotoxic shock, based on methodology from published studies [7] [15].
Materials:
- Z-VAD-FMK (sterile, endotoxin-free)
- Lipopolysaccharide (LPS)
- Phosphate-buffered saline (PBS)
- C57BL/6 mice (6-8 weeks old)
- Equipment for intraperitoneal injection
Procedure:
- Solution preparation: Prepare Z-VAD-FMK in sterile PBS immediately before use.
- Animal groups: Randomly allot mice to control and experimental groups (typically n=5-10 per group).
- Pre-treatment: Administer Z-VAD-FMK via intraperitoneal injection at 5-20 μg/g body weight [7].
- Shock induction: After 2 hours, challenge mice with LPS (10-50 μg/g body weight, i.p.) to induce endotoxic shock.
- Post-treatment analysis:
- Survival monitoring: Record mortality every hour post-LPS challenge [15]
- Serum collection: At 6 hours post-LPS, collect blood for cytokine analysis (TNF-α, IL-6)
- Tissue collection: At 12 hours post-LPS, harvest liver, lungs, and spleen for histopathology
- Peritoneal cell analysis: Collect peritoneal cells for flow cytometric analysis of macrophage populations and necroptosis markers [7]
Research Reagent Solutions
The following table outlines essential materials and reagents for conducting research with Z-VAD-FMK:
Table 3: Essential research reagents for Z-VAD-FMK studies
| Reagent | Function/Application | Specifications |
|---|---|---|
| Z-VAD-FMK | Irreversible pan-caspase inhibitor | >90% purity, CAS 187389-52-2 [11] [14] |
| Anhydrous DMSO | Solvent for stock solutions | Cell culture grade, sterile filtered |
| Camptothecin | Apoptosis inducer (positive control) | 10 μM working concentration [14] |
| Anti-PARP Antibody | Apoptosis validation | Detects full-length (116 kDa) and cleaved (89 kDa) forms [14] |
| Anti-Caspase-3 Antibody | Caspase activation assessment | Detects pro-form and activated fragments |
| LPS | Endotoxic shock induction | Ultrapure, from E. coli serotypes |
| Cell Viability Assay Kits | Cytotoxicity assessment | CCK-8, MTT, or similar assays [7] |
| Flow Cytometry Reagents | Cell death analysis | Annexin V, propidium iodide, anti-CD11b, anti-Gr-1 [15] |
Research Applications and Implications
Elucidating Alternative Cell Death Pathways
The use of Z-VAD-FMK has been instrumental in revealing the existence of alternative backup cell death programs that operate when apoptosis is blocked [16]. Studies utilizing Z-VAD-FMK have demonstrated that caspase inhibition can sensitize cells to necrotic cell death and induce autophagic cell death, highlighting the complex interplay between different cell death modalities [16]. The underlying mechanism of Z-VAD-FMK-mediated sensitization to necrotic cell death involves the inhibition of caspase-8-mediated proteolysis of RIP1 and disturbance of the adenosine nucleotide translocator (ANT)-cyclophilin D (CypD) interaction [16].
In macrophage biology, Z-VAD-FMK pretreatment promotes LPS-induced nitric oxide-mediated necroptosis of bone marrow-derived macrophages, leading to reduced pro-inflammatory cytokine secretion [7] [15]. This effect has significant implications for understanding inflammatory responses, as demonstrated in endotoxic shock models where Z-VAD-FMK treatment alleviates disease pathogenesis by inducing macrophage necroptosis and promoting the accumulation of myeloid-derived suppressor cells (MDSCs) that inhibit macrophage activation [7] [15]. These findings illustrate how caspase inhibition can paradoxically produce protective effects in certain inflammatory contexts by redirecting cell death pathways and modulating immune cell populations.
Limitations and Alternative Interpretations
Despite its utility as a research tool, several limitations and potential misinterpretations must be considered when using Z-VAD-FMK:
Pathway Specificity: Z-VAD-FMK does not inhibit caspase-independent forms of cell death, such as ferroptosis or autophagy [12]. Observed cell death despite Z-VAD-FMK treatment may indicate alternative death mechanisms.
Temporal Considerations: The inhibitor is ineffective against already-activated caspases, primarily blocking zymogen activation [12]. This necessitates early administration before caspase activation cascades commence.
Experimental Artifacts: Improper solubilization (using ethanol or water instead of DMSO) leads to compound precipitation and loss of activity [12]. Long-term storage of DMSO solutions at temperatures above -20°C progressively reduces potency.
Context-Dependent Effects: The role of Z-VAD-FMK-mediated necroptosis in inflammatory disease regulation remains controversial, with both protective and pathogenic outcomes reported across different disease models [16] [15].
The following diagram illustrates the cell death pathway modifications induced by Z-VAD-FMK:
Z-VAD-FMK remains an indispensable research tool for investigating caspase-dependent processes in cell death and inflammation. Its irreversible mechanism of action, targeting the catalytic cysteine residue via the FMK warhead, provides robust inhibition of caspase activation cascades. While its therapeutic application has been limited by toxicity concerns, its role in basic research continues to yield critical insights into cell death pathways and their modulation in disease states [10] [12].
The experimental protocols and parameters outlined in this application note provide researchers with a foundation for employing Z-VAD-FMK in both in vitro and in vivo contexts. However, careful consideration of its limitations—including pathway specificity, temporal requirements, and potential context-dependent effects—is essential for appropriate experimental design and data interpretation. As research continues to evolve, particularly in understanding the crosstalk between different cell death modalities, Z-VAD-FMK will maintain its position as a benchmark tool for distinguishing the unique contributions of caspases in cellular demise and survival pathways.
Application Notes and Protocols
Z-VAD-FMK (carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone) is widely employed in biomedical research as a pan-caspase inhibitor to block apoptotic cell death. Its mechanism involves irreversibly binding to the catalytic cysteine residue within the active site of caspases, thereby preventing the cleavage and activation of downstream substrates [17] [18]. However, a growing body of evidence reveals that its effects extend beyond caspase inhibition, inducing off-target outcomes that complicate data interpretation. These notes detail the inhibitor's specificity, provide validated protocols, and outline critical considerations for its application in mechanistic research and drug development.
Specificity and Selectivity Profile
While Z-VAD-FMK is designated a "pan-caspase" inhibitor, its profile is not exclusive. Its peptide backbone and fluoromethyl ketone (FMK) warhead enable interaction with other cysteine proteases, leading to a complex biological readout.
Table 1: Documented Targets and Off-Target Effects of Z-VAD-FMK
| Target / Effect | Type of Interaction | Functional Outcome | Key Evidence |
|---|---|---|---|
| Caspases (Broad Spectrum) | Primary, Irreversible Inhibition | Inhibition of apoptosis; validation of caspase-dependent pathways [19] [18]. | Widely cited in apoptosis research. |
| N-Glycanase (NGLY1) | Off-target, Irreversible Inhibition | Disruption of ER-associated degradation (ERAD); induction of autophagy [17]. | Needs et al. propose this as the mechanism for Z-VAD-induced autophagy. |
| Caspase-9 | Paradoxical Activation | Amplification of mitochondrial membrane depolarization in certain cell death contexts [20]. | Observed in etoposide-treated mouse embryonic fibroblasts. |
| Necroptosis Induction | Indirect Promotion | Promotion of inflammatory cell death in macrophages upon LPS challenge [15]. | Mediated via caspase-8 inhibition. |
Comparative Analysis with qVD-OPh: The pan-caspase inhibitor qVD-OPh serves as a critical control for distinguishing caspase-specific effects from Z-VAD-FMK's off-target activities [17] [9]. While Z-VAD-FMK potently inhibits NGLY1, qVD-OPh does not, due to its distinct O-phenoxy warhead and quinolyl group [17]. This makes qVD-OPh a superior choice for achieving highly specific caspase inhibition with enhanced cellular permeability and reduced toxicity [18] [9].
Detailed Experimental Protocols
The following protocols are adapted from recent peer-reviewed studies demonstrating key applications and considerations for Z-VAD-FMK.
Protocol: Evaluating Z-VAD-FMK in a Noise-Induced Hearing Loss (NIHL) Model
This in vivo protocol demonstrates the therapeutic application of Z-VAD-FMK to mitigate apoptosis [21].
- Objective: To assess the efficacy of Z-VAD-FMK in protecting cochlear hair cells from noise-induced apoptosis.
- Materials:
- Z-VAD-FMK: Reconstituted in 10% DMSO to a working concentration.
- Animals: Brown Norway rats (or similar model).
- Noise Exposure System: Soundproof chamber with speakers for 110 dB white noise.
- Assessment Tools: Equipment for Auditory Brainstem Response (ABR), facilities for cochlear histology, and Western blot analysis.
- Procedure:
- Pre-Exposure Baseline: Measure baseline ABR thresholds at various frequencies (e.g., 2-32 kHz).
- Noise Trauma: Expose animals to 110 dB SPL octave-band noise for 1 hour.
- Drug Administration: At 6 hours post-exposure, administer a single intraperitoneal injection of Z-VAD-FMK at 3 mg/kg [21].
- Post-Exposure Monitoring: Record ABR thresholds at Days 1, 3, 7, 14, and 28 post-exposure.
- Terminal Analysis: On day 28, harvest cochleae for:
- Immunohistochemistry: Quantify hair cell survival.
- Protein Analysis: Via Western blot to assess cleavage of caspases (e.g., caspase-9) and inflammatory markers (e.g., IL-1β) [21].
- Key Outcomes: Z-VAD-FMK treatment is expected to significantly reduce ABR threshold shifts, decrease hair cell loss, and lower levels of active caspase-9 and IL-1β compared to vehicle-treated controls [21].
Protocol: Investigating Off-Target Autophagy InductionIn Vitro
This cell-based protocol is designed to probe the NGLY1-mediated off-target effect of Z-VAD-FMK [17].
- Objective: To determine if Z-VAD-FMK-induced autophagy is due to caspase inhibition or off-target NGLY1 inhibition.
- Materials:
- Cell Line: Appropriate mammalian cell line (e.g., HeLa, MEFs).
- Inhibitors: Z-VAD-FMK and qVD-OPh.
- Assay Kits: Reagents for monitoring autophagy (e.g., LC3-I/II Western blot, Cyto-ID autophagy assay).
- Antibodies: Anti-LC3, anti-ATG5, anti-ATG7, etc.
- Procedure:
- Cell Seeding: Plate cells in standard culture conditions.
- Treatment Groups:
- Group 1: Vehicle control (e.g., 0.1% DMSO).
- Group 2: Z-VAD-FMK (e.g., 20-80 µM).
- Group 3: qVD-OPh (e.g., 10-50 µM).
- Optional: Positive control for autophagy (e.g., serum starvation).
- Incubation: Treat cells for 24-48 hours.
- Cell Lysis and Analysis:
- Western Blotting: Probe lysates for lipidated LC3-II (a marker of autophagosome formation) and other autophagy-related proteins (ATG3, ATG7). An increase in these proteins upon Z-VAD-FMK but not qVD-OPh treatment indicates an NGLY1-mediated off-target effect [17].
- Viability Assay: Use a CCK-8 or MTT assay concurrently to ensure observed effects are not due to overt cytotoxicity [15].
- Interpretation: Induction of autophagy markers specifically in the Z-VAD-FMK-treated group suggests an NGLY1-dependent mechanism, highlighting the importance of using qVD-OPh as a more specific control.
Signaling Pathway Diagrams
The following diagrams illustrate the primary and secondary pathways modulated by Z-VAD-FMK.
The Scientist's Toolkit: Essential Research Reagents
Table 2: Key Reagents for Caspase Inhibition Studies
| Reagent / Solution | Function / Role | Key Consideration |
|---|---|---|
| Z-VAD-FMK | Broad-spectrum, irreversible caspase inhibitor. Serves as a primary tool for initial apoptosis blocking. | High potential for off-target effects (e.g., NGLY1 inhibition, necroptosis induction). Use requires careful controls [17] [15]. |
| qVD-OPh | Highly specific, broad-spectrum caspase inhibitor. The preferred control for confirming caspase-specific phenomena. | Superior cell permeability and lower toxicity compared to Z-VAD-FMK. Does not inhibit NGLY1 [17] [9]. |
| Ac-DEVD-CHO | Reversible, peptide-based inhibitor of effector caspases (Caspase-3/7). | Useful for in vitro enzymatic assays but has poor cell permeability [18] [9]. |
| Caspase-Glo / DEVD-NucView488 | Luminescent / fluorescent assays for detecting caspase activity in cell populations or via live-cell imaging. | Caspase-Glo is a lytic assay. DEVD-NucView488 is cell-permeable and suitable for real-time, high-content imaging [19]. |
| Lipopolysaccharide (LPS) | TLR4 agonist used to model inflammatory cell death and study crosstalk between apoptosis, pyroptosis, and necroptosis. | In the presence of Z-VAD-FMK, LPS can trigger necroptosis in macrophages, showcasing a key off-target pathway [15]. |
| Etoposide | Chemotherapeutic agent that induces DNA damage and intrinsic apoptosis. | Used in models to study p53-dependent apoptosis and the paradoxical pro-death effects of Z-VAD-FMK [20]. |
Z-VAD-FMK remains a valuable but blunt instrument in the cell biologist's toolkit. Its reputation as a pan-caspase inhibitor is well-earned, but its lack of specificity necessitates rigorous experimental design. For research aimed at conclusively linking a phenotype to caspase activity, the use of qVD-OPh as a complementary inhibitor is strongly recommended to rule out NGLY1-mediated and other off-target effects. Furthermore, researchers should employ multiple lines of evidence, including genetic knockdown of specific caspases and direct activity assays, to build a compelling case. Understanding the full spectrum of Z-VAD-FMK's inhibition is not a limitation but an opportunity to uncover deeper regulatory connections between cell death, protein homeostasis, and inflammatory pathways.
Z-VAD-FMK is a pan-caspase inhibitor that functions as an irreversible covalent inhibitor of caspase proteases. Its mechanism involves the fluoromethyl ketone (FMK) group reacting with the catalytic cysteine residue in the caspase active site, forming a thiomethyl ketone adduct that permanently inactivates the enzyme. While detailed structural studies of Z-VAD-FMK bound specifically to individual caspase active sites are limited in the available literature, its classification as a peptide-based inhibitor with an electrophilic FMK warhead provides fundamental insights into its molecular interactions. This application note details the biochemical protocols for utilizing Z-VAD-FMK in caspase inhibition studies, supplemented by structural data from related caspase-inhibitor complexes.
Caspases are cysteine-dependent aspartate-specific proteases that play critical roles in programmed cell death (apoptosis) and inflammation. Z-VAD-FMK (benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone) represents a broad-spectrum, irreversible caspase inhibitor that has become an essential research tool for investigating apoptotic pathways. As a peptide-based inhibitor, Z-VAD-FMK mimics the natural substrate recognition sequence of caspases but contains a reactive FMK group that covalently modifies the catalytic cysteine residue within the enzyme's active site [9] [22]. This covalent modification permanently inactivates the caspase, effectively blocking its proteolytic activity against natural substrates.
The inhibitor's design capitalizes on the conserved structural features of caspase active sites, which typically recognize tetrapeptide sequences with aspartic acid at the P1 position. The Val-Ala-Asp (VAD) sequence of Z-VAD-FMK provides broad reactivity across multiple caspase family members, while the benzyloxycarbonyl (Z) group at the N-terminus enhances cell permeability, making it suitable for both in vitro and cellular applications [9]. Despite its widespread use, the precise structural determinants of Z-VAD-FMK binding across different caspases remain an active area of investigation, with current understanding derived from its classification within the broader context of caspase inhibitor mechanisms.
Molecular Mechanism of Action
Covalent Inhibition Mechanism
Z-VAD-FMK operates through an irreversible covalent inhibition mechanism directed at the catalytic cysteine residue conserved across caspase family members. The inhibition process involves two critical recognition events:
- Substrate-like binding: The VAD peptide sequence of Z-VAD-FMK binds to the substrate recognition cleft of caspases, positioning the FMK warhead in proximity to the catalytic cysteine residue [9].
- Covalent modification: The fluoromethyl ketone group serves as an electrophilic trap that reacts with the thiol group of the catalytic cysteine, resulting in the formation of a stable thiomethyl ketone covalent adduct [9] [23]. This covalent bond formation permanently inactivates the enzyme by blocking the active site.
The FMK group is particularly effective as a warhead because the fluorine atom serves as an excellent leaving group, facilitating the nucleophilic attack by the cysteine thiolate anion. This reaction mechanism is consistent with other FMK-based protease inhibitors and explains the irreversible nature of Z-VAD-FMK-mediated caspase inhibition.
Structural Context of Caspase Active Sites
Caspases share a conserved fold characterized by a central β-sheet core surrounded by α-helices, with the active site formed at the interface of the large and small subunits in the mature enzyme. The catalytic dyad consisting of cysteine and histidine residues is positioned within a cleft that recognizes tetra-peptide sequences terminating in aspartic acid [24]. Structural studies of related caspase-inhibitor complexes reveal that inhibitor binding typically occurs in a substrate-competitive manner, with the P1 aspartic acid residue forming critical hydrogen bonds with backbone atoms in the S1 pocket [25].
Although detailed crystal structures of Z-VAD-FMK bound to caspases are not available in the searched literature, the inhibitor is expected to occupy the substrate-binding cleft in a manner similar to other peptide-based inhibitors, with the FMK group positioned to react with the catalytic cysteine (Cys285 in caspase-1 numbering). This binding mode would effectively block substrate access while covalently modifying the catalytic nucleophile.
Quantitative Characterization of Inhibition
Biochemical Efficiency Parameters
Table 1: Kinetic Parameters of Caspase Inhibition by Peptide-Based Inhibitors
| Caspase | Inhibitor | IC₅₀ (μM) | Kᵢ (μM) | Mechanism | Reference |
|---|---|---|---|---|---|
| Pan-Caspase | Z-VAD-FMK | Not specified | Not specified | Irreversible | [9] |
| Caspase-6 | S10G | 4.2 | ~2-13 | Allosteric, Non-competitive | [26] |
| Caspase-6 | C13 | 13.2 | ~2-13 | Allosteric, Non-competitive | [26] |
| Caspase-1 | R286A mutant | ~230-fold reduction in kcat/Km | N/A | Disrupted allosteric network | [24] |
| Caspase-1 | E390A mutant | ~130-fold reduction in kcat/Km | N/A | Disrupted salt bridge | [24] |
While specific kinetic parameters for Z-VAD-FMK binding to individual caspases are not provided in the searched literature, its classification as an irreversible inhibitor distinguishes it from the reversible, allosteric inhibitors being developed for caspase-6, which exhibit IC₅₀ values in the low micromolar range [26]. The irreversible nature of Z-VAD-FMK makes classical Michaelis-Menten kinetic parameters such as Kᵢ less meaningful, as the inhibition efficiency depends instead on the second-order rate constant for the inactivation process.
The effectiveness of Z-VAD-FMK as a pan-caspase inhibitor stems from its broad recognition of multiple caspase active sites, though with varying efficiencies across different caspase family members. This contrasts with engineered caspase variants with disrupted allosteric networks (e.g., caspase-1 R286A and E390A mutants) that show dramatically reduced catalytic efficiency due to impaired site-to-site coupling [24].
Research Reagent Solutions
Table 2: Essential Research Reagents for Caspase Inhibition Studies
| Reagent | Function/Description | Application Notes | Storage/Stability |
|---|---|---|---|
| Z-VAD-FMK (unmethylated) | Irreversible pan-caspase inhibitor; essential for active site titration | Critical to use unmethylated derivative for accurate concentration calculations; assumed purity ~95% | Stable in DMSO at -20°C for ≥3 months; stable at room temperature for ≥3 days; survives ≥3 freeze-thaw cycles [23] |
| Ac-DEVD-AFC | Fluorogenic caspase substrate (Caspase-3/7 preference) | Hydrolysis releases fluorescent AFC; used for activity assays | Prepare stock solution in DMSO; store protected from light |
| Ac-VEID-AFC | Fluorogenic caspase substrate (Caspase-6 preference) | Preferred substrate for caspase-6 activity measurements [26] | Prepare stock solution in DMSO; store protected from light |
| Caspase Buffer | Standard reaction buffer (e.g., containing 100 mM NaCl, 50 mM HEPES, 10% sucrose, 0.1% CHAPS, pH 7.4) | Maintains optimal caspase activity and stability | Store at 4°C; supplement with fresh DTT before use |
| Recombinant Caspases | Purified caspase proteins (e.g., caspase-6, caspase-3) | Express in E. coli; purify via Ni²⁺-affinity chromatography [26] [27] | Store in aliquots at -80°C; avoid repeated freeze-thaw cycles |
Experimental Protocols
Protocol 1: Active Site Titration Using Z-VAD-FMK
Purpose: To determine the active concentration of caspase preparations using Z-VAD-FMK as a titration standard.
Materials:
- High-precision analytical balance
- Z-VAD-FMK (unmethylated derivative, ~95% purity)
- Anhydrous DMSO
- Purified caspase solution
- Fluorogenic caspase substrate (e.g., Ac-DEVD-AFC for caspase-3/7, Ac-VEID-AFC for caspase-6)
- Caspase assay buffer
Procedure:
- Z-VAD-FMK Stock Solution Preparation:
- Accurately weigh Z-VAD-FMK using a high-precision balance.
- Calculate the mass based on desired concentration (typically 10 mM), accounting for stated purity (~95%).
- Dissolve in anhydrous DMSO to prepare a 10 mM stock solution.
- Aliquot into 10 μL portions and store at -20°C [23].
Caspase Active Site Titration:
- Prepare a dilution series of the caspase enzyme in assay buffer.
- Incubate each dilution with a molar excess of Z-VAD-FMK (typically 2-5× the estimated caspase concentration) for 30 minutes at room temperature.
- Add fluorogenic substrate and measure residual activity.
- The point at which caspase activity is completely abolished corresponds to the stoichiometric equivalence point between Z-VAD-FMK and active caspase.
Calculation of Active Concentration:
- Active caspase concentration = (Volume of Z-VAD-FMK stock × Concentration of Z-VAD-FMK × Purity factor) / Volume of caspase solution
- Purity factor = Stated purity of Z-VAD-FMK (typically 0.95)
Troubleshooting:
- If incomplete inhibition is observed, increase the Z-VAD-FMK concentration or incubation time.
- Ensure DMSO concentration is consistent across all samples (<1% final concentration).
Protocol 2: Structural Analysis of Caspase-Inhibitor Complexes
Purpose: To investigate the structural basis of caspase inhibition using biophysical and computational approaches.
Materials:
- Purified recombinant caspase
- Inhibitor compounds (Z-VAD-FMK, control inhibitors)
- Crystallization screening kits
- X-ray source or cryo-electron microscope
- Molecular modeling software
Procedure:
- Protein Purification and Complex Formation:
- Express recombinant caspase in E. coli (e.g., BL21(DE3) strain).
- Purify using Ni²⁺-affinity chromatography followed by anion exchange [27].
- Incubate purified caspase with molar excess of Z-VAD-FMK (typically 5-10×) for 1-2 hours at 4°C.
- Remove excess inhibitor using size exclusion chromatography or dialysis.
Structural Determination:
- Crystallographic Approach:
- Computational Approach:
Interaction Analysis:
- Identify hydrogen bonds, salt bridges, and hydrophobic interactions between inhibitor and enzyme.
- Map conformational changes in active site loops and helices (e.g., 60's, 90's, and 130's helices in caspase-6) [28].
- Compare with apo and substrate-bound structures to understand inhibition mechanism.
Applications and Research Implications
Research Applications
Z-VAD-FMK serves as a critical research tool across multiple domains of cell biology and drug discovery:
- Apoptosis Research: Used to distinguish caspase-dependent apoptosis from other forms of cell death. In mouse embryonic fibroblasts, Z-VAD-FMK unexpectedly upregulated caspase-9 cleavage while inhibiting effector caspases (-3, -6, -7), revealing complex feedback mechanisms in apoptotic pathways [29].
- Disease Modeling: Applied in models of acute pancreatitis-associated lung injury, where it demonstrated protective effects by reducing inflammation and apoptosis, highlighting caspases as therapeutic targets [30].
- Target Validation: Employed to confirm caspase involvement in specific pathological processes, such as neurodegeneration linked to caspase-6 activity [26].
- Structural Biology: Serves as a reference compound for comparing inhibition mechanisms across caspase family members, contrasting with allosteric inhibitors that target less conserved regulatory sites [26] [24].
Limitations and Specificity Considerations
While Z-VAD-FMK is widely used as a pan-caspase inhibitor, researchers should consider several important limitations:
- Broad Specificity: The pan-caspase activity of Z-VAD-FMK limits its utility for distinguishing individual caspase contributions to biological processes. Researchers should complement Z-VAD-FMK studies with more specific inhibitors or genetic approaches.
- Cellular Toxicity: FMK-based inhibitors can exhibit toxicity at high concentrations, potentially complicating interpretation of long-term cellular experiments [9].
- Unexpected Effects: Paradoxical pro-apoptotic effects have been observed in some systems, such as enhanced caspase-9 activation in etoposide-treated mouse embryonic fibroblasts [29].
- Alternative Inhibition Strategies: The search for more specific caspase inhibitors has identified allosteric sites as promising targets, such as the putative allosteric pocket on caspase-6 with low sequence conservation among human caspases [26].
Z-VAD-FMK remains a cornerstone reagent for caspase research, providing irreversible inhibition through covalent modification of the catalytic cysteine residue. While detailed structural information on Z-VAD-FMK bound to caspase active sites would enhance our understanding of its binding mode and specificity, its classification as a peptide-based FMK inhibitor places it within a well-characterized class of covalent protease inhibitors. The experimental protocols outlined herein provide robust methodologies for utilizing Z-VAD-FMK in quantitative caspase studies, with applications ranging from basic enzyme characterization to complex cellular models of apoptosis and inflammation. As caspase research advances, Z-VAD-FMK continues to serve as a critical benchmark against which newer, more specific inhibitors are evaluated, particularly those targeting allosteric sites with potential therapeutic advantages.
Caspases are cysteine-dependent proteases that serve as master regulators of multiple programmed cell death (PCD) pathways, including apoptosis, pyroptosis, and necroptosis [1]. The pan-caspase inhibitor Z-VAD-FMK (benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone) has emerged as a critical pharmacological tool for dissecting the complex interplay between these pathways. As an irreversible, cell-permeable inhibitor, Z-VAD-FMK covalently binds to the catalytic site of most caspases, effectively blocking their proteolytic activity [31] [32]. This application note examines the multifaceted cellular consequences of caspase inhibition by Z-VAD-FMK across different PCD pathways and provides detailed protocols for its use in experimental models.
Molecular Mechanisms of Caspase Inhibition by Z-VAD-FMK
Biochemical Properties and Target Specificity
Z-VAD-FMK functions as a broad-spectrum caspase inhibitor that potently inhibits human caspase-1 to -10 with the exception of caspase-2 [31]. It also effectively inhibits murine caspases, notably caspase-1, caspase-3, and caspase-11 [31]. The inhibitor's structure features a fluoromethyl ketone (FMK) group that irreversibly binds to the catalytic cysteine residue in caspases, coupled to a peptide moiety (Val-Ala-Asp) that mimics the caspase cleavage recognition site [9].
Key biochemical properties:
- Molecular Weight: 467.5 g/mol [31] [32]
- Solubility: ≥10 mg/mL in DMSO [31] [32]
- Working Concentration: 5-100 μM for cell culture, typically with 1-hour pretreatment [32]
- Cell Permeability: High, enabling both in vitro and in vivo applications [33]
Differential Effects on Programmed Cell Death Pathways
Z-VAD-FMK exerts distinct effects on different PCD pathways based on their differential dependence on caspase activity:
Table 1: Differential Effects of Z-VAD-FMK on Cell Death Pathways
| Cell Death Pathway | Dependence on Caspases | Effect of Z-VAD-FMK | Key Molecular Players |
|---|---|---|---|
| Apoptosis | High | Strong inhibition | Caspases-3, -8, -9; PARP [1] |
| Pyroptosis | Variable | Context-dependent inhibition | Caspase-1, -4, -5, -11; GSDMD [1] |
| Necroptosis | Negative regulation | Potential promotion | RIPK1, RIPK3, MLKL [7] [1] |
Quantitative Analysis of Z-VAD-FMK Effects Across Experimental Models
Table 2: Quantitative Effects of Z-VAD-FMK in Experimental Models
| Experimental Model | Concentration/Dosage | Key Findings | Mechanistic Insights |
|---|---|---|---|
| Endotoxic Shock Model [7] | 5-20 μg/g body weight | Reduced mortality; decreased pro-inflammatory cytokines (TNF-α, IL-6) | Promoted macrophage necroptosis; enhanced MDSC accumulation |
| Bone Marrow-Derived Macrophages [7] | 20-80 μM | Promoted LPS-induced necroptosis; reduced IL-6 and IL-12 secretion | NO-mediated necroptosis execution |
| Noise-Induced Hearing Loss [21] | 3 mg/kg (single injection) | Mitigated auditory threshold shifts; reduced outer hair cell loss | Decreased caspase-9 and IL-1β levels |
| Cancer Cell Lines [34] | 10 μM | Inhibited staurosporine-induced apoptosis; revealed alternative death pathways | Enabled distinction between caspase-dependent and -independent death |
Experimental Protocols
Protocol: Assessing Z-VAD-FMK in Endotoxic Shock Models
Objective: Evaluate the protective effects of Z-VAD-FMK against LPS-induced endotoxic shock.
Materials:
- Z-VAD-FMK (≥95% purity, dissolved in DMSO)
- LPS (from E. coli or Salmonella)
- C57BL/6 mice (6-8 weeks old)
- ELISA kits for TNF-α, IL-6, IL-12
- Flow cytometry antibodies (CD11b, Gr-1, F4/80)
Procedure:
- Pretreatment: Administer Z-VAD-FMK (5-20 μg/g body weight) or vehicle control via intraperitoneal injection 2 hours before LPS challenge [7].
- Induction of Endotoxic Shock: Inject LPS (10-50 μg/g body weight) intraperitoneally to induce endotoxic shock [7].
- Sample Collection:
- Collect serum samples at 6 hours for cytokine analysis
- Harvest peritoneal cells at 6 and 12 hours for flow cytometry
- Collect liver and lung tissues at 12 hours for histopathology
- Analysis:
- Quantify serum cytokines using ELISA
- Analyze macrophage populations and MDSCs by flow cytometry using CD11b+Gr-1+ markers for MDSCs [7]
- Assess tissue pathology through H&E staining
Expected Results: Z-VAD-FMK pretreatment should significantly reduce mortality, decrease pro-inflammatory cytokine levels, promote peritoneal macrophage necroptosis, and enhance accumulation of MDSCs [7].
Protocol: Distinguishing Cell Death Pathways Using Quantitative Phase Imaging
Objective: Differentiate between apoptosis and primary lytic cell death using Z-VAD-FMK with label-free imaging.
Materials:
- Z-VAD-FMK (10 μM working concentration)
- Cell lines (DU145, LNCaP, or PNT1A)
- Staurosporine (0.5 μM) or doxorubicin (0.1 μM) as cell death inducers
- Quantitative Phase Imaging system (e.g., Q-PHASE)
- CellEvent Caspase-3/7 Green Detection Reagent
- Propidium iodide
Procedure:
- Cell Preparation: Seed cells in μ-Slide I Lauer Family chambers and culture until 70-80% confluent [34].
- Pretreatment: Add Z-VAD-FMK (10 μM) or vehicle control 1 hour before cell death inducers [34].
- Induction of Cell Death: Treat cells with staurosporine (0.5 μM) or doxorubicin (0.1 μM).
- Time-Lapse Imaging:
- Acquire QPI images every 20 minutes for 24-48 hours
- Monitor caspase activation using CellEvent Caspase-3/7 Green (2 μM)
- Assess membrane integrity with propidium iodide (1 μg/mL)
- Data Analysis:
- Extract morphological parameters (cell density, dynamic score)
- Apply machine learning algorithms to classify cell death modalities
- Correlate QPI parameters with fluorescence markers
Expected Results: Z-VAD-FMK should effectively inhibit caspase-3/7 activation and apoptotic morphology induced by staurosporine, but may not prevent primary lytic cell death, allowing distinction between these pathways [34].
Signaling Pathway Diagrams
Z-VAD-FMK Modulation of Programmed Cell Death Pathways
Experimental Workflow for Cell Death Pathway Analysis
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Research Reagents for Z-VAD-FMK Studies
| Reagent / Material | Function / Application | Specifications / Notes |
|---|---|---|
| Z-VAD-FMK [31] [32] | Pan-caspase inhibitor for apoptosis and inflammation studies | ≥95% purity; reconstitute in DMSO (10-20 mM stock) |
| LPS (tlrl-3pelps) [7] [35] | TLR4 agonist for pyroptosis induction and endotoxic shock models | Use at 100 ng/mL for in vitro, 10-50 μg/g for in vivo |
| Staurosporine [35] [34] | Broad-spectrum kinase inducer for intrinsic apoptosis | Working concentration: 0.5-5 μM |
| TNF-α + Z-VAD-FMK [35] | Necroptosis induction combination | TNF-α (50 ng/mL) with Z-VAD-FMK (25 μM) |
| CellEvent Caspase-3/7 [34] | Fluorescent detection of executioner caspase activity | Use at 2 μM for live-cell imaging |
| Propidium Iodide [34] | Membrane integrity indicator for lytic cell death | Use at 1 μg/mL; counterstain with Hoechst 33342 |
| Myeloid-Derived Suppressor Cell Isolation Kit [7] | MDSC purification for immunomodulation studies | Used with CD11b+Gr-1+ markers for mouse MDSCs |
Discussion and Research Implications
The strategic application of Z-VAD-FMK has revealed critical insights into the complex interplay between programmed cell death pathways. While effectively inhibiting apoptosis, Z-VAD-FMK can promote alternative death modalities such as necroptosis under specific conditions, particularly in macrophages exposed to inflammatory stimuli [7]. This paradoxical effect highlights the importance of contextual factors in determining cell fate decisions following caspase inhibition.
Recent research indicates that Z-VAD-FMK may also have off-target effects, including the induction of cellular autophagy through inhibition of N-glycanase NGLY1 rather than caspase inhibition [36]. These findings underscore the necessity of including appropriate controls and complementary assays when interpreting results obtained with this inhibitor.
The translational potential of caspase inhibition is evidenced by studies demonstrating the efficacy of Z-VAD-FMK in disease models ranging from endotoxic shock to noise-induced hearing loss [7] [21]. However, clinical development of caspase inhibitors faces challenges including inadequate efficacy, poor target specificity, and adverse side effects [9]. Future research should focus on developing more specific caspase inhibitors and combination strategies that account for the complex cross-talk between cell death pathways.
Research and Therapeutic Applications: From Bench to Preclinical Models
Caspases are an evolutionarily conserved family of cysteine-dependent proteases that act as crucial regulators of programmed cell death (PCD), mediating pathways including apoptosis, pyroptosis, and necroptosis [1]. The synthetic peptide Z-VAD-FMK (benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone) is a cell-permeant, irreversible pan-caspase inhibitor that effectively prevents induction of apoptosis by binding to the catalytic site of caspase proteases [37] [9]. This application note details the standardized methodologies for utilizing Z-VAD-FMK as a protective agent against apoptosis in various in vitro culture systems, providing researchers with validated protocols and key technical considerations essential for maintaining cellular viability in experimental models.
The efficacy of Z-VAD-FMK stems from its broad-spectrum inhibition of caspase activity. As a peptidomimetic inhibitor, it contains a fluoromethyl ketone (FMK) group that enables irreversible binding to the catalytic cysteine residue in caspases, effectively blocking their proteolytic activity [9]. The O-methylation of the aspartic acid residue at the P1 position enhances the compound's stability and cell permeability, facilitating efficient cellular uptake [37]. Research demonstrates that Z-VAD-FMK's inhibition spans multiple caspases involved in both the initiation (e.g., caspase-8, -9, -10) and execution (e.g., caspase-3, -6, -7) phases of apoptosis, making it particularly valuable for comprehensive apoptosis suppression in diverse experimental contexts [21] [1].
Molecular Mechanism of Action
Caspase Signaling Pathways in Apoptosis
Apoptosis proceeds through two principal signaling pathways that converge on caspase activation. The extrinsic pathway initiates through extracellular death ligands binding to cell surface receptors, leading to the activation of initiator caspases-8 and -10. The intrinsic pathway triggers in response to cellular stress, DNA damage, or developmental cues, causing mitochondrial outer membrane permeabilization and release of cytochrome c, which activates caspase-9 through the apoptosome complex [1]. Both pathways converge on the activation of executioner caspases-3, -6, and -7, which cleave vital cellular substrates including poly-ADP ribose polymerase (PARP), lamin proteins, and other structural components, ultimately leading to characteristic apoptotic morphology and controlled cellular dismantlement [1].
Diagram Title: Z-VAD-FMK Inhibition of Apoptosis Signaling Pathways
Z-VAD-FMK Binding Mechanism
Z-VAD-FMK functions as an irreversible caspase inhibitor through its covalent modification of the catalytic cysteine residue within the caspase active site. The inhibitor's structure comprises three critical domains: the carbobenzoxy (Z) group that enhances cell permeability, the Val-Ala-Asp (VAD) peptide sequence that provides specificity for caspase substrate recognition sites, and the fluoromethyl ketone (FMK) warhead that forms irreversible covalent bonds with thiol groups in catalytic cysteine residues [37] [9]. Structural studies of caspase-6 in complex with Z-VAD-FMK reveal a unique peptide binding mode where the inhibitor occupies the enzyme's active site, preventing substrate access and consequent proteolytic activity [38]. This mechanism effectively halts the caspase cascade at both initiation and execution phases, preserving cellular integrity when apoptosis is induced.
Application Protocols
General Guidelines for Z-VAD-FMK Usage
Z-VAD-FMK is typically supplied as a lyophilized powder or as a solution in DMSO at concentrations ranging from 10-100 mM [37] [39]. For working solutions, reconstitute lyophilized powder in anhydrous DMSO to prepare a 10-20 mM stock solution. Aliquot and store at -20°C with desiccant to maintain stability, avoiding repeated freeze-thaw cycles. The typical effective concentration range for in vitro applications is 5-100 μM, with pretreatment duration of 1 hour prior to apoptosis induction being standard across most cell types [39]. However, optimal concentrations may vary depending on cell type, apoptosis inducer, and exposure duration, necessitating preliminary dose-response studies for specific experimental systems.
Protocol 1: Inhibition of Chemically-Induced Apoptosis
This protocol details the application of Z-VAD-FMK for protecting cells against etoposide-induced apoptosis, validated in mouse embryonic fibroblast (MEF) models [20].
Materials:
- Cell culture system (primary or immortalized MEFs)
- Complete culture medium
- Etoposide stock solution (50-100 mM in DMSO)
- Z-VAD-FMK stock solution (20 mM in DMSO)
- Dimethyl sulfoxide (DMSO) for vehicle control
- Phosphate-buffered saline (PBS)
Procedure:
- Seed cells at appropriate density (e.g., 1×10⁵ cells/mL for MEFs) in complete culture medium and incubate for 24 hours to allow adherence.
- Prepare pretreatment medium containing 50 μM Z-VAD-FMK in complete culture medium. For vehicle control, add equivalent DMSO volume (typically ≤0.5% v/v).
- Replace existing medium with pretreatment medium and incubate cells for 1 hour at 37°C, 5% CO₂.
- While cells are pretreating, prepare treatment medium containing both 50 μM Z-VAD-FMK and 50 μg/mL etoposide.
- After pretreatment, carefully remove pretreatment medium and replace with treatment medium.
- Incubate cells for 48 hours at 37°C, 5% CO₂.
- Assess apoptosis inhibition through appropriate methodologies such as flow cytometry with Annexin V/PI staining, caspase activity assays, or Western blot analysis of caspase cleavage and PARP processing.
Key Considerations:
Protocol 2: Enhancing Cryopreservation Recovery of Stem Cells
This protocol describes the use of Z-VAD-FMK to improve post-thaw viability of human embryonic stem cells (hESCs) during cryopreservation processes [40].
Materials:
- hESC cultures
- Standard freezing solution (e.g., containing DMSO)
- Z-VAD-FMK stock solution (100 mM in DMSO)
- Complete hESC culture medium
- Cryogenic vials
- Controlled-rate freezing apparatus
Procedure:
- Prepare freezing solution supplement with 100 μM Z-VAD-FMK.
- Harvest intact hESC colonies using standard methodology, ensuring maintenance of colony integrity.
- Resuspend cells in freezing solution containing Z-VAD-FMK.
- Aliquot cell suspension into cryogenic vials and proceed with controlled-rate freezing according to standard protocols.
- Store vials in liquid nitrogen until required.
- For thawing, rapidly warm cryovials and transfer cell suspension to pre-warmed culture medium.
- Centrifuge gently to remove cryoprotectant and resuspend in fresh culture medium supplement with 100 μM Z-VAD-FMK.
- Plate cells and maintain in Z-VAD-FMK supplemented medium for 24-48 hours post-thaw.
- Assess cell survival using MTT assay or similar viability measurement at 24-48 hours post-thaw.
Key Considerations:
- Applying Z-VAD-FMK in both freezing solution and post-thaw culture medium provides significantly enhanced survival rates (18.7%) compared to freezing solution alone (10.2%) or vehicle control (9.9%) [40].
- Z-VAD-FMK exposure does not significantly enhance spontaneous differentiation of hESC within post-thaw culture, maintaining pluripotency [40].
Protocol 3: Protection Against Ischemic/ Hypoxic Stress
This protocol applies to protecting granulosa cell lines under serum starvation and hypoxic conditions, modeling ischemic stress in ovarian tissue transplantation [41].
Materials:
- Granulosa cell lines (e.g., GC1a, HGL5, COV434)
- Standard and serum-free culture media
- Z-VAD-FMK stock solution (20 mM in DMSO)
- Hypoxia chamber or tri-gas incubator (capable of maintaining 1% O₂)
Procedure:
- Seed cells at density of 1.0×10⁴ (GC1a, HGL5) or 2.5×10⁴ (COV434) cells per well in 96-well plates.
- Incubate for 24 hours at 37°C, 5% CO₂ to allow adherence.
- Prepare serum-free medium containing 50 μM Z-VAD-FMK.
- Replace standard medium with serum-free medium containing Z-VAD-FMK.
- Transfer cells to tri-gas incubator maintaining 1% O₂, 5% CO₂, and balance N₂.
- Incubate cells under hypoxic conditions for 48 hours.
- Assess metabolic activity using WST-1 assay or similar methodology, and viability through FACS analysis with Annexin V/PI staining.
Key Considerations:
- Unlike chemical apoptosis induction, Z-VAD-FMK may not provide significant protective effects against hypoxia/serum starvation-induced damage in granulosa cell lines, highlighting the importance of cell death mechanism in inhibitor efficacy [41].
- Under these conditions, modifications in expression of apoptosis-related molecules (p53, Bax, Bcl-xl, PARP) may not be observed despite Z-VAD-FMK treatment [41].
Quantitative Effectiveness Data
Table 1: Summary of Z-VAD-FMK Efficacy Across Experimental Models
| Cell System | Apoptosis Inducer | Z-VAD-FMK Concentration | Treatment Duration | Key Outcomes | Reference |
|---|---|---|---|---|---|
| Human granulosa cell lines (GC1a, HGL5, COV434) | Etoposide (50 μg/mL) | 50 μM | 48 hours | Protected against etoposide-induced cell death; maintained metabolic activity | [41] |
| Human Embryonic Stem Cells (hESC) | Cryopreservation stress | 100 μM | In freezing solution + 24-48 hours post-thaw | Enhanced post-thaw survival rate to 18.7% vs 9.9% in controls | [40] |
| Mouse Embryonic Fibroblasts (MEFs) | Etoposide (50 μg/mL) | 50 μM | 48 hours | Increased loss of ΔΨm and caspase-9 cleavage despite inhibition of effector caspases | [20] |
| Jurkat cells | Anti-Fas mAb | 20 μM | Varies with experiment | Effective inhibition of apoptosis induction | [37] |
Table 2: Z-VAD-FMK Preparation and Storage Specifications
| Parameter | Specification | Notes |
|---|---|---|
| Molecular Weight | 467.5 g/mol | [39] |
| Purity | >95% | HPLC analysis |
| Solubility | Soluble in DMSO at 5 mg/mL (~10.7 mM) | [39] |
| Stock Solution Stability | 24 months lyophilized at -20°C | Store desiccated |
| Reconstituted Solution Stability | 3 months at -20°C | Aliquot to avoid freeze-thaw cycles |
| Working Concentration Range | 5-100 μM | Cell-type dependent |
| Standard Pretreatment Time | 1 hour | Prior to apoptosis induction |
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Materials for Z-VAD-FMK Apoptosis Inhibition Studies
| Reagent/Equipment | Function/Application | Specifications |
|---|---|---|
| Z-VAD-FMK | Irreversible pan-caspase inhibitor | 20-100 mM stock in DMSO; cell-permeant |
| DMSO (Dimethyl sulfoxide) | Solvent vehicle for reagent preparation | Anhydrous, tissue culture grade; maintain final concentration ≤0.5% |
| Etoposide | DNA-damaging apoptosis inducer | 50-100 mM stock in DMSO; working concentration 50 μg/mL |
| Anti-Fas mAb | Extrinsic pathway apoptosis inducer | Working concentration varies by cell type |
| Annexin V-FITC/PI Apoptosis Detection Kit | Flow cytometry-based apoptosis quantification | Dual staining for early (Annexin V+) and late (Annexin V+/PI+) apoptosis |
| WST-1/MTT Assay Kits | Metabolic activity measurement | Colorimetric assessment of cell viability |
| Caspase Activity Assay Kits | Direct caspase activation measurement | Fluorometric or colorimetric substrates |
| Hypoxia Chamber/Tri-gas Incubator | Induction of hypoxic/ischemic stress | Capable of maintaining 1% O₂, 5% CO₂, balance N₂ |
| Controlled-Rate Freezing Apparatus | Standardized cryopreservation | For stem cell preservation studies |
Experimental Workflow Integration
Diagram Title: Z-VAD-FMK Experimental Workflow
Critical Considerations and Limitations
While Z-VAD-FMK serves as a valuable tool for apoptosis inhibition, researchers must consider several important limitations. Paradoxical effects have been documented where Z-VAD-FMK unexpectedly amplifies certain aspects of cell death signaling. In mouse embryonic fibroblasts, Z-VAD-FMK increased etoposide-induced mitochondrial membrane depolarization (ΔΨm loss), cytochrome c release, and caspase-9 cleavage and activity despite effectively inhibiting effector caspases (-3, -6, -7) [20]. Similar amplification of p53-dependent apoptosis has been observed in rat embryonic fibroblasts, indicating that these paradoxical effects may be cell-type and context dependent.
The protective efficacy of Z-VAD-FMK varies significantly across different apoptosis inducers. While it demonstrates robust protection against chemical inducers like etoposide and receptor-mediated apoptosis via Fas activation, it may provide limited protection against ischemia/hypoxia-induced cell death, as observed in granulosa cell lines under hypoxic conditions with serum starvation [41]. This highlights the importance of considering alternative cell death pathways that may operate independently of caspase activation.
Researchers should also note that Z-VAD-FMK exhibits inhibitory activity beyond caspases, including effects on cathepsin B, PNGase, and picornaviral 2A proteinases [39]. These off-target effects should be considered when interpreting experimental results, particularly in systems where these enzymes play significant roles. Additionally, Z-VAD-FMK has been shown to inhibit mitogen-induced T cell proliferation, indicating potential impacts on cellular functions beyond apoptosis regulation [39].
For applications requiring extended treatment durations or in vivo translation, researchers should consider alternative caspase inhibitors such as Q-VD-OPh, which demonstrates enhanced efficacy, permeability, and reduced toxicity profiles compared to Z-VAD-FMK [9]. However, it is noteworthy that Q-VD-OPh also shares some paradoxical effects with Z-VAD-FMK, including increased etoposide-induced loss of ΔΨm and caspase-9 cleavage in MEF models [20].
Within the complex pathophysiology of sepsis and endotoxic shock, dysregulated cell death is a critical driver of excessive inflammation and subsequent immune suppression. The pan-caspase inhibitor Z-VAD-FMK has emerged as a valuable research tool for investigating these processes. As a cell-permeable, irreversible caspase inhibitor, it facilitates the study of apoptotic pathways and reveals intriguing crossover effects with other cell death modalities. This application note synthesizes current research findings and provides detailed protocols for utilizing Z-VAD-FMK in experimental models of endotoxic shock and sepsis, framed within the broader context of caspase inhibition research for inflammatory conditions.
Quantitative Profiling of Z-VAD-FMK Activity
Z-VAD-FMK exhibits broad-spectrum inhibition against caspase family members with varying efficiency. The table below summarizes its inhibitory profile and key biochemical characteristics.
Table 1: Biochemical and Inhibition Profile of Z-VAD-FMK
| Parameter | Description / Value |
|---|---|
| Common Names | Z-VAD-FMK; Z-Val-Ala-Asp(OMe)-fluoromethylketone; z-VAD-fmk [42] |
| Inhibitor Class | Halomethylketone cysteine peptidase inhibitor [42] |
| Mechanism | Irreversible inhibition via reaction with the active site cysteine [42] |
| Molecular Weight | 467.5 g/mol [43] |
| Recommended Storage | -20°C, desiccated (lyophilized or in DMSO solution) [43] |
| Key Caspase Inhibition (Half-time at 1 µM) | Caspase-1: 2.5 s; Caspase-3: 43 s; Caspase-8: 2.5 s; Caspase-9: 3.9 s [42] |
| Other Reported Inhibitory Activities | Cathepsin B, Cathepsin H, Rhinovirus picornain 2A, Peptide:N-glycanase (PNGase) [42] [43] |
| Pharmaceutical Relevance | Not suitable for drug use due to metabolism producing toxic fluoroacetate [42] |
Experimental Applications and Protocols
In Vivo Application: Murine Model of Endotoxic Shock
This protocol is adapted from studies demonstrating that Z-VAD-FMK alleviates endotoxic shock in mice [7] [15].
Materials
- Animals: Female C57BL/6 mice (6-8 weeks old) [7] [15].
- Inducing Agent: Lipopolysaccharide (LPS) [7] [15].
- Test Article: Z-VAD-FMK [7] [15].
- Vehicle: Saline or DMSO (ensure final DMSO concentration in administration is safe for animals) [7] [15].
Procedure
- Pre-treatment: Administer Z-VAD-FMK intraperitoneally (5-20 µg per gram of body weight) 2 hours before LPS challenge [7] [15].
- Challenge: Induce endotoxic shock by intraperitoneal injection of a high dose of LPS (10-50 µg per gram of body weight). The specific dose determines the mortality rate for survival studies [7] [15].
- Post-treatment Analysis:
- Serum Collection: Collect serum at 6 hours post-LPS challenge to measure pro-inflammatory cytokines (e.g., TNF-α, IL-6) [7] [15].
- Tissue Collection: Harvest organs (liver, lung, spleen) at 12 hours for histopathological analysis [7] [15].
- Peritoneal Cells: Collect peritoneal cells at 6 and 12 hours for flow cytometric analysis of cell death and population dynamics (e.g., macrophages, MDSCs) [7] [15].
- Survival Monitoring: For survival studies, monitor mice every hour following high-dose LPS injection [7] [15].
Key Findings from this Model
Treatment with Z-VAD-FMK significantly reduces mortality and alleviates tissue pathology in LPS-challenged mice. The protective mechanism is associated with:
- Promotion of nitric oxide-mediated necroptosis in macrophages, reducing their numbers [7] [15].
- Inhibition of pro-inflammatory cytokine secretion from macrophages [7] [15].
- Promotion of myeloid-derived suppressor cell (MDSC) accumulation, which further suppresses macrophage activation [7] [15].
In Vitro Application: Macrophage Studies
This protocol is used to investigate the direct effects of Z-VAD-FMK on bone marrow-derived macrophages (BMDMs) or peritoneal macrophages [7] [15].
Materials
- Cells: Bone marrow-derived macrophages (BMDMs) or thioglycollate-elicited peritoneal macrophages [7] [15].
- Cell Culture Medium: Complete DMEM supplemented with GM-CSF (10 ng/ml) for BMDMs [7] [15].
- Stimulant: LPS (100 ng/ml) [7] [15].
- Test Article: Z-VAD-FMK (typically 20-80 µM for BMDMs, 5-45 µM for peritoneal macrophages) [7] [15].
Procedure
- Cell Preparation: Generate BMDMs from mouse bone marrow cultured for 7 days with GM-CSF, or harvest peritoneal macrophages 3 days after thioglycollate injection [7] [15].
- Pre-treatment: Pre-incubate cells (1 × 10⁵ cells per well in a 96-well plate) with Z-VAD-FMK for 30 minutes [7] [15].
- Stimulation: Challenge cells with LPS (100 ng/ml) and culture for 24-48 hours [7] [15].
- Analysis:
In Vitro Application: Apoptosis Inhibition Control
Z-VAD-FMK is widely used as a control to confirm caspase-dependent apoptosis. The following protocol is a generalized example.
Materials
- Cells: Any relevant cell line (e.g., Jurkat, H9c2, primary cells) [44] [45].
- Apoptosis Inducer: Campthothecin, Etoposide, or other inducers [41] [45].
- Test Article: Z-VAD-FMK (often used at 20-50 µM) [41] [45].
Procedure
- Pre-treatment: Pre-incubate cells with 20-50 µM Z-VAD-FMK for 30 minutes to 2 hours [41] [45] [43].
- Induction: Treat cells with the apoptosis inducer (e.g., 4 µM campthothecin for Jurkat cells for 3 hours) [45].
- Analysis:
- Apoptosis Quantification: Stain cells with Annexin V and Propidium Iodide (PI) followed by flow cytometry. Z-VAD-FMK should significantly reduce the Annexin V-positive population [45].
- Western Blotting: Analyze cleavage of apoptotic markers like PARP, Caspase-3, and Caspase-8. Pre-treatment with Z-VAD-FMK should prevent this cleavage [41] [46].
Signaling Pathways and Mechanisms
Z-VAD-FMK's role in inflammatory models is complex and involves crosstalk between different cell death pathways.
Diagram 1: Z-VAD-FMK modulates cell death and inflammation. By inhibiting caspase-8, Z-VAD-FMK blocks apoptosis but can promote necroptosis. In endotoxic shock, this leads to macrophage necroptosis and MDSC accumulation, which collectively inhibit pro-inflammatory M1 macrophage activation and reduce the overall inflammatory response [7] [15] [47].
The Scientist's Toolkit: Essential Research Reagents
Table 2: Key Reagents for Z-VAD-FMK Research in Inflammation Models
| Reagent / Kit | Function / Application | Example Usage |
|---|---|---|
| Z-VAD-FMK | Irreversible, cell-permeable pan-caspase inhibitor. Core investigative compound. | Used in vitro (5-100 µM) and in vivo (5-20 µg/g mouse) to inhibit caspase activity [7] [15] [43]. |
| Lipopolysaccharide (LPS) | Pathogen-associated molecular pattern (PAMP) used to model endotoxic shock and sepsis. | In vivo: 10-50 µg/g for shock models. In vitro: 100 ng/ml to stimulate macrophages [7] [15]. |
| Annexin V / PI Apoptosis Detection Kit | Flow cytometry-based kit to distinguish apoptotic (Annexin V+/PI-) and necrotic (Annexin V+/PI+) cells. | Confirm apoptosis inhibition by Z-VAD-FMK in control experiments [45]. |
| Cell Viability Assay (e.g., CCK-8, WST-1) | Colorimetric assay to measure metabolic activity as a proxy for cell viability. | Assess overall cell health and death in response to LPS ± Z-VAD-FMK [41] [7]. |
| Cytokine ELISA Kits | Quantify specific protein levels of cytokines (e.g., TNF-α, IL-6) in cell culture supernatant or serum. | Measure the inflammatory response and its modulation by Z-VAD-FMK [7]. |
| Antibodies for Western Blot | Detect cleavage of apoptosis markers (e.g., Caspase-3, PARP) and other signaling proteins. | Validate caspase inhibition and study cell death pathways [41] [46]. |
| Myeloid-Derived Suppressor Cell (MDSC) Isolation Kit | Immunomagnetic separation of MDSCs from mouse spleen or blood. | Isolate MDSCs to study their role in Z-VAD-FMK-mediated protection [7] [15]. |
Caspases, an evolutionarily conserved family of cysteine-dependent proteases, serve as master regulators of programmed cell death (PCD) and inflammation, playing fundamental roles in cellular homeostasis and disease pathogenesis [1]. Dysregulation of caspase-mediated pathways is implicated in diverse conditions, including neurological disorders, hearing loss, cancer, and inflammatory diseases [1] [9]. Among therapeutic strategies, caspase inhibition has emerged as a promising neuroprotective approach. Z-VAD-FMK (benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone), a pan-caspase inhibitor, has demonstrated significant efficacy in preclinical models, particularly in protecting auditory function against noise-induced and ototoxic damage [21] [48]. This application note details the mechanistic basis, experimental protocols, and quantitative findings supporting the therapeutic potential of Z-VAD-FMK, providing a structured resource for researchers and drug development professionals.
Caspase Functions and Signaling Pathways in Cell Death
Caspases are categorized functionally as initiators (caspases-2, -8, -9, -10) or executioners (caspases-3, -6, -7) of apoptosis, and as inflammatory caspases (caspases-1, -4, -5, -11, -12) [1] [9]. They orchestrate multiple PCD pathways:
- Apoptosis: The intrinsic (mitochondrial) pathway involves caspase-9 activation via the apoptosome, leading to executioner caspase activation. The extrinsic (death receptor) pathway is initiated by caspase-8. Executioner caspases dismantle the cell by cleaving structural and repair proteins like PARP [1].
- Pyroptosis: Inflammatory caspases (e.g., caspase-1) cleave gasdermin proteins (e.g., GSDMD), generating N-terminal fragments that form plasma membrane pores, resulting in lytic, inflammatory cell death and IL-1β release [1].
- Necroptosis: This caspase-independent pathway can be inhibited by caspase-8, which cleaves key necroptosis components like RIPK1 and RIPK3 [1].
The following diagram illustrates the complex interplay between caspases and these key programmed cell death pathways:
Figure 1. Caspase-Mediated Programmed Cell Death Pathways. This diagram illustrates the core signaling pathways in apoptosis, pyroptosis, and necroptosis, highlighting the central role of specific caspases as initiators, executioners, and molecular switches. Dashed lines represent key regulatory interactions and cross-talk between pathways.
Quantitative Analysis of Z-VAD-FMK Efficacy in Hearing Loss Models
Z-VAD-FMK has been quantitatively evaluated in multiple hearing loss models. The data below summarize its protective effects on auditory function and cochlear histology.
Table 1. Efficacy of Z-VAD-FMK in Noise-Induced Hearing Loss (NIHL) Rodent Model [21]
| Evaluation Parameter | Noise-Exposed Group Results | Noise + Z-VAD-FMK Group Results | Protective Effect |
|---|---|---|---|
| ABR Threshold Shift | Permanent threshold shift across all frequencies, minimal recovery by day 28 | Significantly mitigated threshold shifts, particularly at low and mid frequencies | Partial but significant functional protection |
| ABR Wave I Amplitude | Diminished amplitude at suprathreshold levels (80 dB) | Significantly mitigated amplitude reduction | Preservation of auditory nerve output |
| Outer Hair Cell Survival | Significant loss across middle and basal cochlear turns | Significant rescue of outer hair cells | Direct cytoprotection in sensory epithelium |
| Molecular Markers | Elevated caspase-9 and IL-1β levels | Reduced caspase-9 and IL-1β levels | Inhibition of apoptosis and inflammatory pyroptosis |
Table 2. Efficacy of Z-VAD-FMK in Ototoxic (Actinomycin-D) Hearing Loss Model [48]
| Evaluation Parameter | Act-D Exposed Group Results | Act-D + Z-VAD-FMK Group Results | Protective Effect |
|---|---|---|---|
| Hair Cell Loss | Significant, dose- and time-dependent hair cell loss and apoptosis | Significantly reduced hair cell loss and apoptosis | Direct protection against ototoxic damage |
| Spiral Ganglion Neurons (SGNs) | No obvious damage to SGNs or auditory nerve fibers (ANFs) | No significant change from Act-D alone | Specific protection targeted to hair cells |
| Mechanism | Activation of caspase-mediated apoptosis in hair cells | Inhibition of cysteine proteases; increased cell survival | Pan-caspase inhibition |
Table 3. Context-Dependent and Unexpected Effects of Z-VAD-FMK [41] [20]
| Experimental Context | Observed Effect of Z-VAD-FMK | Interpretation & Clinical Implication |
|---|---|---|
| Etoposide-induced cell death (MEFs) | Increased mitochondrial membrane depolarization (loss of ΔΨm), cytochrome c release, and caspase-9 cleavage/activity | Context-dependent pro-death effect: Suggests a complex feedback loop; underscores need for careful pre-clinical modeling. |
| Ischemic conditions (Granulosa cells) | No protective effect under hypoxia and serum starvation | Lack of universal efficacy: Efficacy may be dependent on specific death triggers and cellular metabolic state. |
| Normoxic conditions (Granulosa cells) | Protection from etoposide-induced cell death | Context-dependent pro-life effect: Confirms efficacy in standard apoptosis models. |
Detailed Experimental Protocols
Protocol: In Vivo Efficacy of Z-VAD-FMK in Noise-Induced Hearing Loss
This protocol is adapted from the 2025 rodent model study that demonstrated the efficacy of Z-VAD-FMK against permanent noise-induced hearing loss [21].
1. Experimental Groups and Animal Model
- Animals: Brown Norway rats (15-17 weeks old), equal numbers of males and females.
- Group Allocation (n=8/group):
- Group 1: Unexposed control
- Group 2: Noise-exposed
- Group 3: Noise + vehicle (10% DMSO)
- Group 4: Noise + Z-VAD-FMK (3 mg/kg)
2. Noise Exposure Procedure
- Acoustic Trauma: Continuous octave-band noise (4–8 kHz) at 110 dB SPL for 1 hour.
- Calibration: Use a calibrated sound level meter to ensure uniform sound distribution (<2 dB variation) within the soundproof chamber.
- Animal Monitoring: Monitor animals at 15-minute intervals during noise exposure while kept awake and unrestrained.
3. Drug Administration
- Compound: Z-VAD-FMK (commercially available from TOCRIS, Cat #2163).
- Preparation: Dilute in 10% DMSO. Prepare immediately before use and store at -20°C until administration.
- Dosage and Route: Administer a single intraperitoneal (i.p.) injection at 3 mg/kg.
- Timing: Administer 6 hours post conclusion of noise exposure.
4. Functional Assessment: Auditory Brainstem Response (ABR)
- Timeline: Record ABRs pre-exposure and at days 1, 3, 7, 14, and 28 post-intervention.
- Anesthesia: Anesthetize animals using a cocktail of ketamine (44 mg/kg, i.m.) and xylazine (5 mg/kg, i.m.), maintaining body temperature at 37°C.
- Electrode Placement: Position subdermal needle electrodes at the vertex (active), ventrolateral to the tested ear (reference), and near the hind leg (ground).
- Stimuli and Recording:
- Frequencies: 2, 4, 8, 16, 24, and 32 kHz.
- Intensity: Present stimuli from 90 dB down to threshold in 5 dB steps.
- Threshold Determination: Identify the lowest intensity at which reproducible Wave I and IV peaks are visually detected.
- Suprathreshold Analysis: At 80 dB SPL, measure Wave I peak amplitude (node-to-peak) and latency.
5. Histological and Molecular Analysis
- Terminal Endpoint: Euthanize animals on day 28 post-intervention via cardio-perfusion.
- Cochlear Harvesting and Processing:
- Harvest cochleae and fix in 4% paraformaldehyde for 48 hours.
- Decalcify in 10% EDTA for approximately 3 weeks.
- For immunostaining, permeabilize with 0.3% Triton-X in PBS and block with 5% normal horse serum.
- Hair Cell Quantification: Stain with hair cell-specific markers (e.g., Myosin VIIa). Count surviving outer and inner hair cells along the entire basilar membrane under a fluorescence microscope.
- Protein Analysis: For Western blot, harvest cochlear tissues 24 hours post-intervention. Analyze protein levels of key markers like cleaved caspase-9 and IL-1β.
The workflow for this comprehensive in vivo protocol is summarized below:
Figure 2. In Vivo Protocol Workflow for Assessing Z-VAD-FMK in NIHL. This diagram outlines the key stages of the rodent model protocol, from baseline assessment and noise exposure to long-term functional and histological evaluation.
Protocol: In Vitro Assessment of Z-VAD-FMK on Cochlear Hair Cells
This protocol evaluates the direct protective effect of Z-VAD-FMK on hair cells in explanted cochlear cultures exposed to ototoxic agents [48].
1. Cochlear Organotypic Culture
- Tissue Source: Isplicate organs of Corti from postnatal day 3 (P3) Sprague-Dawley rats.
- Culture Medium: Maintain explants in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 100 U/mL penicillin.
- Conditions: Culture at 37°C in a humidified atmosphere of 5% CO₂.
2. Ototoxic Insult and Drug Treatment
- Ototoxin: Apply Actinomycin-D (Act-D) at concentrations ranging from 5 to 20 μM for 24-48 hours.
- Z-VAD-FMK Co-treatment: Co-apply Z-VAD-FMK at a specified concentration (e.g., 50-100 μM based on pilot studies) simultaneously with Act-D.
- Control Groups: Include untreated controls, Act-D only, and Z-VAD-FMK only groups.
3. Hair Cell Survival Analysis
- Fixation and Staining: Fix cultures in 4% paraformaldehyde and stain with phalloidin (for F-actin in stereocilia) and anti-Myosin VIIa antibody (for hair cell cytoplasm).
- Imaging and Quantification: Capture images using confocal microscopy. Count surviving inner and outer hair cells per 100 μm length of the basilar membrane in predefined regions (apex, middle, base).
- Apoptosis Assay: Perform TUNEL staining or immunostaining for activated caspase-3 to quantify apoptotic cells.
The Scientist's Toolkit: Key Research Reagent Solutions
Table 4. Essential Reagents for Z-VAD-FMK-Mediated Neuroprotection Research
| Reagent / Resource | Specifications & Source Examples | Primary Function in Research |
|---|---|---|
| Z-VAD-FMK (Pan-caspase Inhibitor) | TOCRIS (Cat #2163); water-soluble preparations available. Broad-spectrum, irreversible inhibitor. | Core therapeutic agent; inhibits initiator and executioner caspases by binding catalytic cysteine residue. |
| Vehicle Control Solution | 10% DMSO in physiological saline or PBS. | Control for drug delivery; critical for ruling out solvent-mediated effects. |
| Actinomycin-D (Act-D) | Commercial chemotherapy agent; transcription inhibitor. | Induces intrinsic apoptosis pathway in in vitro ototoxicity models (e.g., hair cell cultures). |
| Etoposide | Commercial topoisomerase II inhibitor. | Standard apoptotic inducer for in vitro models (e.g., granulosa cells, MEFs) to test efficacy. |
| Antibodies for Immunoblotting | Anti-caspase-9, anti-cleaved caspase-3, anti-IL-1β, anti-PARP. | Detection of target engagement (caspase cleavage) and downstream apoptotic/inflammatory activity. |
| Cochlear Hair Cell Markers | Anti-Myosin VIIa, Phalloidin (F-actin stain). | Histological identification and quantification of hair cell survival and structural integrity. |
| Apoptosis Detection Kits | TUNEL assay kits; Annexin V-FITC/PI flow cytometry kits. | Confirmation of apoptotic cell death and evaluation of inhibitor efficacy. |
The compelling preclinical data for Z-VAD-FMK, particularly in hearing loss models, underscores the therapeutic potential of caspase inhibition in neuroprotection. The successful application notes and protocols provided herein demonstrate a clear pathway from mechanistic understanding to in vivo and in vitro validation. However, the transition of caspase inhibitors like Z-VAD-FMK to clinical use faces significant challenges, including potential context-dependent effects and the need for exquisite target specificity to avoid interfering with non-apoptotic caspase functions [20] [9]. Future research should focus on optimizing delivery strategies, such as local intracochlear administration for hearing disorders, to minimize systemic exposure [21]. Furthermore, combination therapies targeting parallel damage pathways (e.g., antioxidants alongside apoptosis inhibitors) may yield synergistic benefits. As our understanding of caspase biology evolves beyond apoptosis and inflammation, so too will the opportunities for developing sophisticated, effective, and safe caspase-targeted neuroprotective therapies.
Atherosclerosis, a major underlying cause of cardiovascular disease, is characterized by the accumulation of apoptotic cells within atherosclerotic plaques, contributing significantly to plaque instability and rupture [49]. The morphological features of apoptosis include cell shrinkage, chromatin condensation, and eventual disintegration into apoptotic bodies, which are typically cleared by macrophages through efferocytosis [49]. In advanced atherosclerotic lesions, however, defective clearance of these apoptotic cells leads to secondary necrosis, enlargement of the necrotic core, and sustained inflammation, ultimately accelerating plaque progression and vulnerability [49].
Caspases, a family of cysteine-dependent aspartate-specific proteases, function as central regulators of programmed cell death (PCD), including apoptosis, pyroptosis, and necroptosis [1]. These enzymes are intricately controlled through epigenetic modifications, molecular interactions, and post-translational changes, positioning them as critical mediators of cellular homeostasis and disease pathogenesis [1]. Dysregulation of caspase-mediated pathways is implicated in a wide array of pathological conditions, including cancer, neurodegenerative disorders, and inflammatory diseases such as atherosclerosis [1].
The pan-caspase inhibitor benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone (z-VAD-FMK) is a permeable synthetic peptide that irreversibly binds to the catalytic site of caspases, thereby inhibiting apoptosis and other caspase-dependent cell death pathways [41]. Research demonstrates that z-VAD-FMK can protect granulosa cells from etoposide-induced death under normoxic conditions, confirming its efficacy as a caspase inhibitor [41]. However, its protective role appears context-dependent, as it fails to prevent cell death induced by serum starvation and hypoxia [41]. In macrophage models, z-VAD-FMK induces alternative non-apoptotic cell death pathways, such as autophagy and necrosis, an effect potentially mediated by receptor-interacting protein 1 (RIP1) and associated with a pronounced inflammatory response through the secretion of cytokines like TNFα [50]. This highlights both the possibilities and limitations of z-VAD-FMK for therapeutic interventions aimed at stabilizing vulnerable atherosclerotic plaques [50].
Table 1: Key Caspases in Programmed Cell Death Relevant to Atherosclerosis
| Caspase | Primary Role | Main Pathways | Relevance to Atherosclerosis |
|---|---|---|---|
| Caspase-8 | Initiator | Extrinsic apoptosis, necroptosis switch, pyroptosis via GSDMC cleavage [1] | Regulates death receptor-mediated apoptosis in vascular cells [49] |
| Caspase-9 | Initiator | Intrinsic (mitochondrial) apoptosis [1] | Activates executioner caspases in response to cellular stress [49] |
| Caspase-3/-7 | Executioner | Apoptosis execution, PARP cleavage, pyroptosis via GSDME cleavage [1] | Final effectors dismantling cells; markers of apoptosis in plaques [49] |
| Caspase-1 | Inflammatory | Pyroptosis via GSDMD cleavage, inflammasome activation [1] | Promotes inflammatory cell death in plaques |
Mechanism of Action of z-VAD-FMK
Biochemical Properties
z-VAD-FMK is a cell-permeable, irreversible broad-spectrum caspase inhibitor. Its structure features a benzyloxycarbonyl (Z) group that enhances membrane permeability, a Val-Ala-Asp (VAD) peptide sequence that confers specificity for caspase active sites, and a fluoromethyl ketone (FMK) group that covalently binds to the catalytic cysteine residue in the caspase active site, permanently inhibiting enzyme activity [41] [51]. The inhibitor exhibits high specificity for caspases due to the conserved requirement among caspases for aspartic acid in the P1 position of their substrates [51].
Molecular Interactions and Signaling Consequences
Within the apoptotic cascade, z-VAD-FMK potently inhibits initiator caspases (such as caspase-8 and -9) and executioner caspases (such as caspase-3 and -7) [41]. By preventing caspase activation, z-VAD-FMK blocks the proteolytic cleavage of key cellular substrates, including poly (ADP-ribose) polymerase (PARP), thereby preserving DNA repair mechanisms and preventing the characteristic morphological changes of apoptosis [41]. However, caspase inhibition can shift cell fate towards alternative death pathways. In macrophages, z-VAD-FMK treatment induces RIP1-dependent autophagy and necrotic cell death, accompanied by significant secretion of proinflammatory cytokines like TNFα, which can exacerbate local inflammation and indirectly induce smooth muscle cell necrosis, potentially undermining plaque stability [50].
Diagram 1: z-VAD-FMK mechanism in apoptosis signaling.
Application Notes: z-VAD-FMK in Atherosclerosis Research
Cell-Type-Specific Effects in Atherosclerotic Plaques
The efficacy and consequences of z-VAD-FMK treatment vary significantly across different cell types present in atherosclerotic plaques:
- Macrophages/Foam Cells: z-VAD-FMK induces RIP1-dependent autophagy and necrotic cell death in macrophages, accompanied by secretion of proinflammatory cytokines (e.g., TNFα) and chemokines [50]. This differential sensitivity is largely attributed to higher expression levels of RIP1 in macrophages compared to other vascular cells [50].
- Vascular Smooth Muscle Cells (VSMCs): VSMCs are relatively resistant to z-VAD-FMK-induced direct cytotoxicity. However, they can undergo necrosis indirectly when exposed to the combination of z-VAD-FMK and TNFα secreted by treated macrophages [50].
- Endothelial Cells: Similar to VSMCs, endothelial cells demonstrate greater resistance to z-VAD-FMK-induced non-apoptotic death compared to macrophages [50].
Implications for Plaque Stability
The potential therapeutic application of z-VAD-FMK for atherosclerotic plaque stabilization must be carefully considered:
- Potential Benefit: Selective induction of non-apoptotic death in plaque macrophages could theoretically reduce macrophage content and inflammation in lesions [50].
- Significant Limitations: The accompanying inflammatory response and indirect induction of SMC death are detrimental effects. As VSMCs are crucial for maintaining the fibrous cap integrity, their loss promotes plaque instability and rupture risk [50].
Experimental Protocols
In Vitro Assessment of z-VAD-FMK on Macrophage Cell Death
4.1.1 Objective: To evaluate the concentration-dependent effects of z-VAD-FMK on macrophage cell death and cytokine secretion.
4.1.2 Materials:
- Macrophage cell lines (e.g., J774A.1, RAW264.7) or primary mouse peritoneal macrophages
- z-VAD-FMK (typically prepared as 20-50 mM stock solution in DMSO)
- Cell culture medium appropriate for macrophage type
- Etoposide (for apoptosis induction control)
- WST-1 assay kit for metabolic activity measurement
- Annexin V-FITC/PI apoptosis detection kit for flow cytometry
- ELISA kits for TNFα detection
4.1.3 Methodology:
- Cell Culture and Treatment:
- Culture macrophages in appropriate medium supplemented with 10% FBS.
- Seed cells in 96-well plates (1.0×10⁴ cells/well for metabolic assays) or 6-well plates (for flow cytometry).
- Pre-treat cells with z-VAD-FMK at concentrations ranging from 10-100 µM for 1-2 hours.
- For apoptosis induction controls, treat cells with etoposide (50 µg/ml) with or without z-VAD-FMK co-treatment.
- Incubate cells for 24-48 hours under normoxic conditions (20% O₂, 5% CO₂, 37°C).
Metabolic Activity Assessment:
- Add WST-1 solution (10 µl/100 µl medium) to each well.
- Incubate for 1-4 hours at 37°C.
- Measure absorbance at 450 nm with reference wavelength at 620 nm.
- Calculate metabolic activity relative to untreated controls.
Cell Death Analysis:
- Harvest cells by gentle scraping.
- Wash twice with cold PBS.
- Resuspend in binding buffer and stain with Annexin V-FITC and PI according to manufacturer's protocol.
- Analyze by flow cytometry within 1 hour.
Cytokine Measurement:
- Collect cell culture supernatants by centrifugation.
- Analyze TNFα secretion using commercial ELISA kits according to manufacturer's instructions.
4.1.4 Expected Results: z-VAD-FMK treatment should inhibit etoposide-induced apoptosis but increase necrotic cell death (Annexin V-/PI+ or Annexin V+/PI+ populations) in macrophages. This should be accompanied by increased TNFα secretion in culture supernatants [50].
Hypoxia/Serum Starvation Model Simulating Ischemic Conditions
4.2.1 Objective: To investigate z-VAD-FMK effects under conditions mimicking post-transplantation ischemia or plaque hypoxia.
4.2.2 Methodology:
- Culture macrophages as described in protocol 4.1.3.
- Replace complete medium with serum-free medium.
- Transfer cells to hypoxic chamber (1% O₂, 5% CO₂, 94% N₂ at 37°C).
- Treat cells with z-VAD-FMK (50 µM) or vehicle control.
- Incubate for 48 hours under hypoxic conditions.
- Assess metabolic activity, cell viability, and apoptosis-related molecules (p53, Bax, Bcl-xl, PARP) by Western blot.
4.2.3 Expected Results: Under hypoxic/serum starvation conditions, z-VAD-FMK may not provide significant protection against cell death, and expressions of apoptosis-related molecules may not be substantially modulated [41].
Table 2: Experimental Conditions for z-VAD-FMK Treatment in Macrophage Models
| Experimental Condition | z-VAD-FMK Concentration | Treatment Duration | Key Readouts | Expected Outcome |
|---|---|---|---|---|
| Normoxia + Etoposide | 50 µM | 48 hours | Annexin V/PI staining, PARP cleavage | Inhibition of apoptosis, shift to necrosis [41] |
| Normoxia (basal) | 10-100 µM | 24-48 hours | Metabolic activity (WST-1), TNFα secretion | Concentration-dependent cell death, inflammation [50] |
| Hypoxia (1% O₂) + Serum Starvation | 50 µM | 48 hours | Metabolic activity, p53, Bax, Bcl-xl expression | Limited protective effect [41] |
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Research Reagents for Studying Cell Death in Atherosclerosis
| Reagent/Cell Line | Function/Application | Key Characteristics |
|---|---|---|
| z-VAD-FMK | Pan-caspase inhibitor | Irreversible, cell-permeable, targets active site cysteine [41] [51] |
| J774A.1/RAW264.7 Cells | Macrophage models | Mouse macrophage lines sensitive to z-VAD-induced non-apoptotic death [50] |
| Primary Mouse Peritoneal Macrophages | Primary macrophage model | Requires IFN-γ priming for z-VAD-FMK sensitivity [50] |
| Etoposide | Apoptosis inducer | DNA topoisomerase II inhibitor; positive control for apoptosis induction [41] |
| WST-1 Assay | Metabolic activity measurement | Measures mitochondrial dehydrogenase activity; indicator of cell viability [41] |
| Annexin V-FITC/PI Apoptosis Kit | Cell death discrimination | Distinguishes apoptotic (Annexin V+/PI-) from necrotic (Annexin V+/PI+) cells [41] |
Data Interpretation and Analysis
Quantitative Analysis of Cell Death Modalities
When interpreting experimental results with z-VAD-FMK, researchers should quantitatively analyze the distribution of cell death modalities:
- Apoptotic Population: Annexin V+/PI- cells should decrease with z-VAD-FMK treatment when apoptosis is induced.
- Necrotic Population: Annexin V-/PI+ and Annexin V+/PI+ populations typically increase with z-VAD-FMK treatment in macrophages.
- Metabolic Activity: WST-1 assay results may show reduced metabolic activity despite inhibition of apoptosis, indicating alternative death pathways.
Optimization Guidelines
- Concentration Optimization: Perform dose-response curves with z-VAD-FMK (10-100 µM) to determine optimal concentration for specific cell types.
- Time-Course Analysis: Conduct time-response experiments (24-72 hours) to track temporal progression of cell death pathways.
- Combination Treatments: Test z-VAD-FMK in combination with death receptor ligands or other cell death inducers to explore pathway interconnections.
Diagram 2: Experimental workflow for z-VAD-FMK analysis.
Z-VAD-FMK is a cell-permeable, irreversible pan-caspase inhibitor that non-selectively binds to the catalytic site of caspase enzymes, effectively halting the initiation and execution phases of apoptosis [21] [31]. Its broad-spectrum inhibitory activity encompasses multiple caspases involved in both inflammatory and apoptotic pathways, making it a valuable tool for researching programmed cell death (PCD) across various disease models [52] [31]. This document provides a comprehensive summary of effective Z-VAD-FMK concentrations and detailed administration protocols across different experimental systems, serving as a practical resource for researchers designing studies on caspase-mediated mechanisms.
The effective concentration of Z-VAD-FMK varies significantly depending on the experimental model, route of administration, and specific pathological context. The tables below summarize the empirically validated dosages and concentrations from recent research.
Table 1: In Vivo Dosage and Administration in Animal Models
| Disease Model | Species | Dosage | Route | Timing | Key Efficacy Findings | Source |
|---|---|---|---|---|---|---|
| Noise-Induced Hearing Loss | Rat | 3 mg/kg | Intraperitoneal (i.p.) | Single dose, 6 hours post-noise exposure | Mitigated ABR threshold shifts, reduced hair cell loss, decreased caspase-9 & IL-1β [21] | |
| Endotoxic Shock | Mouse | 5-20 μg/g | Intraperitoneal (i.p.) | Pre- or post-treatment (2h) from LPS challenge | Reduced mortality, alleviated disease, promoted macrophage necroptosis [7] | |
| Myocardial Infarction | Rat | 1 mg/kg | Intraperitoneal (i.p.) | Daily for 32 days | Improved cardiac function, mitigated pathological remodeling, reduced apoptosis [53] | |
| Fuchs Endothelial Corneal Dystrophy | Mouse | 0.1% eye drops | Topical | Twice daily from 8 to 28 weeks | Higher endothelial cell density, improved hexagonality [54] |
Table 2: In Vitro Working Concentrations in Cell Culture
| Cell Type/Line | Concentration Range | Incubation Time | Primary Purpose | Key Observations | Source |
|---|---|---|---|---|---|
| Human Corneal Endothelial Cells (FECD model) | 10 μM | 24 hours | Suppress TGF-β-induced cell death & ECM production | Reduced apoptosis and ECM accumulation [54] | |
| Bone Marrow-Derived Macrophages (BMDMs) | 20 - 80 μM | 30 min pre-treatment before LPS | Induce necroptosis, inhibit pro-inflammatory cytokines | Promoted LPS-induced necroptosis, reduced cytokine secretion [7] | |
| Primary T Cells | Not specified (non-toxic doses) | Varies | Inhibit proliferation | Suppressed T cell proliferation, independent of caspase inhibition [55] | |
| General Cell Culture (various lines) | 10 - 100 μM | 1 - 48 hours (context-dependent) | Inhibit apoptosis | Blocks caspase activity and features of apoptosis [56] |
Detailed Experimental Protocols
Protocol for Noise-Induced Hearing Loss (NIHL) Rodent Model
This protocol is adapted from a study demonstrating the efficacy of a single dose of Z-VAD-FMK in protecting against permanent hearing loss after acoustic trauma [21].
Materials:
- Brown Norway rats (15-17 weeks old)
- Z-VAD-FMK (e.g., TOCRIS R&D Systems, Cat #2163)
- Vehicle: 10% DMSO
- Anesthesia: Ketamine (44 mg/kg) and Xylazine (5 mg/kg)
- Auditory Brainstem Response (ABR) equipment
Procedure:
- Noise Exposure: Expose awake, unrestrained rats to octave-band noise (4-8 kHz, 110 dBA) for 1 hour.
- Drug Preparation and Administration:
- Prepare a fresh solution of Z-VAD-FMK in 10% DMSO.
- At 6 hours post-noise exposure, administer a single intraperitoneal (i.p.) injection at a dosage of 3 mg/kg.
- Include control groups: unexposed, noise-exposed only, and noise + vehicle.
- Functional Assessment:
- Measure Auditory Brainstem Responses (ABRs) prior to noise exposure (baseline) and at days 1, 3, 7, 14, and 28 post-exposure.
- Anesthetize rats using a ketamine/xylazine cocktail.
- Record ABR thresholds at frequencies from 2 kHz to 32 kHz.
- Tissue Collection and Analysis:
- Euthanize animals at day 28 post-intervention.
- Harvest cochleae for immunohistochemical analysis of hair cell survival and protein-level analysis (e.g., caspase-9, IL-1β).
Protocol for In Vitro Caspase Inhibition in Macrophages
This protocol outlines the use of Z-VAD-FMK to study caspase inhibition and its effects on macrophage cell death and inflammation, as applied in endotoxic shock research [7].
Materials:
- Bone Marrow-Derived Macrophages (BMDMs) or peritoneal macrophages
- Z-VAD-FMK (e.g., Beyotime Biotechnology)
- Lipopolysaccharide (LPS)
- Cell culture medium and standard reagents
Procedure:
- Cell Preparation:
- Isolate and differentiate BMDMs from mouse tibias and femurs using GM-CSF (10 ng/ml) for 7 days.
- Alternatively, harvest peritoneal macrophages by lavage 3 days after thioglycollate injection.
- Drug Treatment and Stimulation:
- Pre-treat macrophages with Z-VAD-FMK (e.g., 20-80 μM for BMDMs, 5-45 μM for peritoneal macrophages) for 30 minutes.
- Stimulate cells with LPS (100 ng/ml) for the desired duration.
- Assessment of Outcomes:
- Cell Viability: Use CCK-8 assay after 48 hours to measure viability.
- Necroptosis: Analyze by flow cytometry for propidium iodide (PI) uptake.
- Cytokine Secretion: Measure levels of pro-inflammatory cytokines (e.g., TNF-α, IL-6) in the supernatant via ELISA or qPCR.
- Nitric Oxide (NO) Production: Quantify NO levels in the culture medium.
Signaling Pathways and Experimental Workflows
Z-VAD-FMK in Programmed Cell Death Pathways
The following diagram illustrates the central role of caspases in key programmed cell death (PCD) pathways and the inhibitory points of Z-VAD-FMK.
Experimental Workflow for In Vivo Efficacy Testing
This workflow outlines the key steps for evaluating Z-VAD-FMK in an animal model of disease, such as noise-induced hearing loss or myocardial infarction.
The Scientist's Toolkit: Essential Research Reagents
The table below lists key reagents and materials commonly used in Z-VAD-FMK research, as referenced in the cited studies.
Table 3: Essential Research Reagents and Materials
| Reagent/Material | Function/Application | Example Specifications / Notes |
|---|---|---|
| Z-VAD-FMK | Irreversible, pan-caspase inhibitor. Core research molecule. | Purity ≥95% [31] [56]. Soluble in DMSO (e.g., 10-20 mM stock). Aliquots stable at -20°C for months. |
| Vehicle (DMSO) | Solvent for reconstituting Z-VAD-FMK. | Use high-grade, sterile DMSO. Final concentration in vivo should be minimized (e.g., 3% V/V or less) [53]. |
| LPS (Lipopolysaccharide) | Induces inflammatory signaling and endotoxic shock models. | Used at 100 ng/ml in vitro [7] or 10-50 μg/g in vivo to model endotoxic shock. |
| Anesthesia Cocktail | For in vivo procedures (surgery, ABR measurements). | Ketamine (44 mg/kg) + Xylazine (5 mg/kg) i.m. used for ABR in rodents [21]. |
| Cell Viability Assay Kits | Quantify cell survival/death after treatment. | CCK-8 assay [7] or Annexin V/PI staining for apoptosis/necroptosis detection. |
| Antibodies for Protein Analysis | Detect molecular changes via Western Blot, IHC. | Targets: Cleaved Caspases (e.g., Casp-3, -9), IL-1β, pMLKL, RIPK3 [21] [53]. |
Challenges, Limitations, and Protocol Optimization for Z-VAD-FMK
Caspase-8 represents a critical regulatory node at the intersection of multiple cell death pathways. While traditionally recognized for its role in initiating apoptosis, emerging research reveals that caspase-8 inhibition triggers a paradoxical switch to necroptosis—a form of programmed necrosis with distinct morphological and immunological consequences. This application note examines the mechanisms underlying this cell death transition and provides detailed protocols for studying necroptosis induction via caspase-8 inhibition, with particular focus on the pan-caspase inhibitor zVAD-fmk and its specialized counterpart z-IETD-fmk.
The paradoxical effect of caspase inhibition stems from the physiological role of caspase-8 as a suppressor of necroptotic signaling. Under normal conditions, caspase-8 cleaves and inactivates key necroptosis mediators including RIPK1 and RIPK3 [57]. Pharmacological inhibition disrupts this regulatory function, thereby unleashing the necroptotic program [58]. This phenomenon has significant implications for therapeutic applications, ranging from anti-infection strategies to concerns about the safety of caspase inhibitors in clinical trials [59].
Experimental Data: Quantitative Analysis of Necroptosis Induction
Table 1: Experimental Models of zVAD-fmk-Induced Necroptosis
| Cell Type/Model | Treatment Conditions | Key Findings | Cell Death Percentage | Citation |
|---|---|---|---|---|
| Primary hepatocytes | TNF-α/ActD + ZVAD-fmk | Apoptosis blocked at 24h; necrosis at 48h | Significant necrosis at 48h | [59] |
| L929 mouse fibrosarcoma | zVAD-fmk alone | Necroptosis dependent on autocrine TNFα production | Dose-dependent increase | [58] |
| Classically activated macrophages (CAMs) | LPS + zVAD-fmk | Necroptosis via ROS-mediated MLKL/p38 activation | Rapidly induced | [60] |
| In vivo mouse model | LPS/GalN + ZVD-fmk | Early protection diminished by necrosis switch | Protection lost by 48h | [59] |
| Endotoxic shock model | zVAD-fmk pretreatment | Reduced mortality despite macrophage necroptosis | 80-90% survival vs 10-20% controls | [7] |
Table 2: Comparative Analysis of Caspase Inhibitors in Cell Death Modulation
| Inhibitor | Specificity | Primary Effect | Secondary Consequences | Therapeutic Implications |
|---|---|---|---|---|
| zVAD-fmk | Pan-caspase | Apoptosis inhibition | Necroptosis induction | Safety concerns in clinical trials [59] |
| z-IETD-fmk | Caspase-8 preferential | Necroptosis induction | Enhanced bacterial clearance | Potential anti-infective application [57] |
| Necrostatin-1 | RIPK1 inhibition | Necroptosis blockade | Partial protection | Context-dependent efficacy [59] |
| z-YVAD-fmk | Caspase-1 inhibition | Inflammasome suppression | No significant effect on bacterial clearance | Limited efficacy in infection models [57] |
Signaling Mechanisms: From Caspase Inhibition to Necroptosis Execution
The molecular pathway connecting caspase-8 inhibition to necroptosis execution involves a carefully orchestrated sequence of events. In susceptible cell types, caspase-8 inhibition prevents the proteolytic inactivation of RIPK1 and RIPK3, allowing these kinases to form a amyloid signaling complex known as the necrosome [58] [57]. This complex then phosphorylates the terminal necroptosis effector MLKL, leading to its oligomerization and translocation to plasma membranes where it induces membrane disruption [60].
The transcriptional regulation of this process involves protein kinase C-mediated activation of MAPKs and the transcription factor AP-1, which drives TNFα transcription in an autocrine loop [58]. Additionally, reactive oxygen species serve as critical amplifiers of this pathway by enhancing MLKL and p38 activation [60]. In classically activated macrophages, zVAD-fmk induces necroptosis through ROS-mediated activation of both MLKL and p38, creating a feed-forward loop that potentiates the necroptotic signal [60].
Figure 1: Molecular Pathway of Caspase-8 Inhibition-Induced Necroptosis. This diagram illustrates the key signaling events from caspase inhibition to membrane disruption and inflammatory response.
Experimental Protocols: Methodologies for Necroptosis Research
Protocol 1: Induction and Quantification of Necroptosis in Murine Macrophages
Primary Objective: To evaluate zVAD-fmk-induced necroptosis in classically activated macrophages (CAMs).
Materials & Reagents:
- J774.1/JA-4 macrophage cell line or primary bone marrow-derived macrophages
- Lipopolysaccharide (LPS from E. coli O55:B5, 100 ng/mL)
- zVAD-fmk (pan-caspase inhibitor, 20-80 μM)
- Necrostatin-1 (RIPK1 inhibitor, 10-30 μM)
- Butylated hydroxyanisole (ROS inhibitor, 50-100 μM)
- Cell Counting Kit-8 (CCK-8) for viability assessment
- Phospho-MLKL and total MLKL antibodies for immunoblotting
- ROS detection reagents (e.g., DCFDA or CellROX)
Procedure:
- Macrophage Activation: Culture macrophages in complete medium and stimulate with LPS (100 ng/mL) for 20 hours to induce classical activation [60].
- Inhibitor Treatment: Pre-treat CAMs with necroptosis modulators (Nec-1 or BHA) for 30 minutes, then add zVAD-fmk (20-80 μM) for specified durations [60].
- Viability Assessment:
- Harvest cells after treatment periods (3-24 hours)
- Incubate with CCK-8 reagent for 1 hour at 37°C
- Measure absorbance at 450nm using a microplate reader
- Calculate percentage viability relative to untreated controls [7]
- Mechanistic Analysis:
- For ROS detection: Incubate cells with ROS-sensitive fluorescent dyes during treatment
- For signaling analysis: Lyse cells in RIPA buffer, separate proteins by SDS-PAGE, transfer to PVDF membranes, and immunoblot with phospho-specific antibodies [59]
- Morphological Assessment:
- Stain cells with Hoechst 33342 (5 μg/mL) and propidium iodide (1 μg/mL) for 10 minutes
- Visualize using fluorescence microscopy
- Identify necrotic cells as PI-positive with no apoptotic nuclear condensation [59]
Validation Parameters:
- Significant cell death within 3-6 hours of zVAD-fmk treatment
- Death suppression by Nec-1 but not apoptotic inhibitors
- Increased MLKL phosphorylation detected by immunoblotting
- ROS generation preceding cell death
Protocol 2: In Vivo Analysis of Caspase Inhibition in Endotoxic Shock
Primary Objective: To assess the therapeutic and paradoxical effects of caspase inhibition in murine endotoxic shock models.
Materials & Reagents:
- C57BL/6 mice (6-8 weeks old)
- LPS (E. coli serotype, 10-50 μg/g body weight)
- D-galactosamine (GalN, 700 mg/kg) for sensitization
- zVAD-fmk or z-IETD-fmk (5-20 μg/g body weight)
- Serum collection tubes and EDTA-coated capillaries
- Cytokine/chemokine detection arrays or ELISA kits
Procedure:
- Experimental Groups: Randomly allocate mice to control and treatment groups (n=5-8 per group) [7].
- Inhibitor Administration: Administer caspase inhibitors intraperitoneally 30 minutes before or 2 hours after LPS challenge [7] [57].
- Sample Collection:
- Collect serum samples at 6 hours post-LPS challenge for cytokine analysis
- Harvest organs (liver, lung, spleen) at 12 hours for histology
- Collect peritoneal lavage fluid for immune cell analysis [7]
- Inflammatory Response Analysis:
- Survival Studies: Monitor mice every hour for 24-48 hours following high-dose LPS challenge (25-50 μg/g) [7].
Validation Parameters:
- Reduced mortality in zVAD-fmk treated mice despite cellular necroptosis
- Decreased pro-inflammatory cytokines in serum
- Enhanced bacterial clearance in infection models with z-IETD-fmk
- Temporal switch from apoptosis to necrosis in liver injury models
Figure 2: Experimental Workflow for Necroptosis Induction Studies. This diagram outlines the key steps in designing and executing experiments to study caspase inhibition-mediated necroptosis.
The Scientist's Toolkit: Essential Reagents for Necroptosis Research
Table 3: Research Reagent Solutions for Necroptosis Studies
| Reagent/Category | Specific Examples | Function/Application | Experimental Considerations |
|---|---|---|---|
| Caspase Inhibitors | zVAD-fmk (pan-caspase), z-IETD-fmk (caspase-8 preferential) | Induce necroptosis by blocking caspase-8 mediated RIPK1/RIPK3 cleavage | Cell-type specific responses; concentration optimization required [58] [57] |
| Necroptosis Inhibitors | Necrostatin-1 (RIPK1 inhibitor), MLKL inhibitors | Confirm necroptosis mechanism; assess pathway specificity | Variable efficacy across cell types; use multiple concentrations [59] [60] |
| Cell Death Detection | CCK-8 viability assay, Hoechst 33342/PI staining, LDH release | Quantify and characterize cell death modality | Combine multiple assays for accurate death classification [59] [7] |
| Signaling Analysis | Phospho-specific antibodies (RIPK1, RIPK3, MLKL, p38) | Monitor pathway activation through immunoblotting | Time-course experiments essential for pathway elucidation [60] |
| ROS Detection | DCFDA, CellROX, MitoSOX | Measure reactive oxygen species generation | Critical for mechanistic studies in macrophages [60] |
| In Vivo Models | LPS/GalN-induced liver injury, endotoxic shock, bacterial infection | Assess physiological relevance of necroptosis | z-IETD-fmk shows therapeutic potential in infection models [59] [57] |
Discussion: Research Applications and Therapeutic Implications
The paradoxical induction of necroptosis via caspase inhibition presents both challenges and opportunities for therapeutic development. Safety concerns regarding caspase inhibitors in clinical trials are substantiated by findings demonstrating that caspase inhibition blocks early apoptosis but triggers delayed necrosis, ultimately diminishing protective effects [59]. This phenomenon is particularly relevant in liver injury models where caspase inhibitors initially protect against apoptosis but subsequently promote necrotic cell death with inflammatory consequences.
Conversely, the strategic induction of necroptosis through caspase-8 inhibition demonstrates therapeutic potential in bacterial infection models. z-IETD-fmk treatment promotes neutrophil recruitment and enhances bacterial clearance without direct antimicrobial effects, suggesting host-directed therapeutic applications [57]. This approach represents a promising strategy for combating antibiotic-resistant infections without exerting selective pressure on pathogens.
The cell-type specificity of this paradoxical cell death warrants careful consideration. While macrophages undergo zVAD-fmk-induced necroptosis through ROS-mediated activation of MLKL and p38 [60], neutrophils respond to caspase-8 inhibition through a RIPK3- and IFN-β-dependent pathway that enhances inflammatory responses without cell death [57]. These differential responses highlight the importance of cellular context in determining the outcome of caspase inhibition.
Future research directions should focus on developing more selective caspase-8 inhibitors with improved therapeutic windows, identifying biomarkers to predict cell death outcomes in specific pathological contexts, and exploring combination therapies that harness the beneficial effects of necroptosis while minimizing detrimental consequences.
Caspases are an evolutionarily conserved family of cysteine-dependent proteases that play central roles in mediating programmed cell death (PCD), including apoptosis, pyroptosis, and necroptosis [1] [9]. As key executors of cellular demise, their activity is tightly regulated through epigenetic modifications, molecular interactions, and post-translational changes [1]. The dysregulation of caspase-mediated pathways is implicated in a wide array of pathological conditions, including cancer, neurodegenerative disorders, and inflammatory diseases, establishing them as potential therapeutic targets [1] [61] [9].
Z-VAD-FMK (carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone) is a cell-permeant, irreversible pan-caspase inhibitor that binds to the catalytic site of caspase proteases [37] [62]. This compound functions as a broad-spectrum apoptosis inhibitor by covalently linking with the nucleophilic active thiol site of caspases, effectively preventing proteolytic activity [9] [63]. As a peptide-based inhibitor modified with a fluoromethyl ketone group, Z-VAD-FMK exhibits improved cell permeability and stability compared to earlier caspase inhibitors [9]. Its mechanism primarily involves blocking the activation of pro-caspases rather than directly inhibiting already-activated enzymes, thereby preventing the caspase-dependent cleavage of cellular substrates that lead to apoptotic morphology [63].
The therapeutic potential of Z-VAD-FMK spans multiple disease contexts, yet its effects demonstrate significant variability depending on cellular context, stimulus type, and experimental conditions. This application note synthesizes current research findings on the context-dependent outcomes of Z-VAD-FMK treatment, providing structured experimental data, detailed protocols, and visual frameworks to guide research applications.
Molecular Mechanisms and Signaling Pathways
Caspase Functions in Programmed Cell Death
Caspases execute diverse roles across different PCD pathways. In apoptosis, caspases-2, -8, -9, and -10 function as initiators, while caspases-3, -6, and -7 act as executioners [1] [61]. The intrinsic (mitochondrial) pathway involves caspase-9 and is regulated by Bcl-2 family proteins, while the extrinsic pathway is initiated by caspase-8 via death receptor signaling [61]. In pyroptosis, inflammatory caspases (caspases-1, -4, -5, -11) cleave gasdermin proteins, generating N-terminal fragments that form plasma membrane pores, leading to inflammatory cell death [1]. Caspase-8 serves as a molecular switch between apoptosis, necroptosis, and pyroptosis, with its inhibition potentially shifting cell fate between these pathways [1].
Z-VAD-FMK inhibits this broad spectrum of caspase-mediated processes by targeting the conserved catalytic site, though its efficacy varies significantly across different PCD pathways and cellular contexts [64] [9].
Visualizing Caspase Pathways and Z-VAD-FMK Inhibition
The following diagram illustrates the key programmed cell death pathways and the intervention point of Z-VAD-FMK:
Diagram 1: Caspase-Mediated Cell Death Pathways and Z-VAD-FMK Inhibition. This diagram illustrates the major programmed cell death pathways regulated by caspases and the strategic inhibition point of Z-VAD-FMK across initiator, inflammatory, and executioner caspases.
Context-Dependent Outcomes of Z-VAD-FMK Treatment
Quantitative Analysis of Variable Cellular Responses
The efficacy of Z-VAD-FMK in preventing cell death varies substantially across different cell types, death stimuli, and experimental conditions. The table below synthesizes quantitative findings from multiple studies:
Table 1: Context-Dependent Effects of Z-VAD-FMK Across Experimental Models
| Cell Type/Model | Stimulus | ZVAD Treatment | Outcome | Key Metrics | Reference |
|---|---|---|---|---|---|
| Brown Norway rats | Noise-induced hearing loss (110 dB, 1h) | 3 mg/kg, i.p., 6h post-noise | Partial protection | ≈50% reduction in threshold shifts; outer hair cell rescue | [21] |
| Human BM-MSCs | FAS ligation + SMAC-mimetic | 20-100 µM | Significant apoptosis inhibition | ~80% reduction in cell death | [64] |
| Human BM-MSCs | TNFα + SMAC-mimetic + Q-VD-OPh | 20-100 µM | Minimal protection | ~25% cell death despite inhibition | [64] |
| Human BM-MSCs | BH3 mimetics (BCL-2/-xL/MCL-1 inhibition) | Not specified | No protection | BAX/BAK-dependent apoptosis proceeds | [64] |
| Drosophila S2 cells | SMN depletion via RNAi | Concentration not specified | Reversed apoptosis | Caspase-dependent death prevented | [65] |
| L929 murine fibrosarcoma | TNFα + zVAD-fmk | 20-100 µM | Enhanced necrosis | Apoptosis-to-necrosis shift | [61] |
| Jurkat T cells | FAS ligation | 20 µM | Strong apoptosis inhibition | >90% protection | [64] [63] |
Critical Factors Influencing Z-VAD-FMK Efficacy
Cell Type-Specific Variations:
- Mesenchymal Stromal Cells (MSCs): Human MSCs demonstrate relative resistance to extrinsic apoptosis induced by FAS ligation, with only ~20-40% cell death even at high agonist concentrations [64]. This resistance is overcome by combining FAS activation with IAP antagonism, resulting in robust, Z-VAD-FMK-inhibitable cell death [64].
- Neuronal Cells: In noise-induced hearing loss models, Z-VAD-FMK partially protects cochlear hair cells, particularly outer hair cells across middle and basal turns, with associated reductions in caspase-9 and IL-1β levels [21].
- Immune Cells: Jurkat T cells show high sensitivity to Z-VAD-FMK protection from FAS-mediated apoptosis, while primary T cells exhibit dose-dependent inhibition of proliferation [64] [63].
Stimulus-Dependent Responses:
- Death Receptor vs. Mitochondrial Activation: Z-VAD-FMK effectively blocks death receptor-mediated apoptosis but shows limited efficacy against BAX/BAK-driven intrinsic apoptosis when pro-survival BCL-2 proteins are directly inhibited [64].
- Inflammatory Stimuli: The compound demonstrates variable effects on pyroptosis pathways, potentially related to its ability to inhibit inflammatory caspases at different concentrations [1] [9].
- Metabolic Stressors: Shifts in intracellular ATP levels can alter Z-VAD-FMK effectiveness, with energy depletion favoring necrosis over apoptosis [61].
Pathway Compensation and Shift Mechanisms: When apoptosis is effectively inhibited by Z-VAD-FMK, cells may undergo alternative death pathways. In L929 murine fibrosarcoma cells, TNFα stimulation in the presence of Z-VAD-FMK shifts cell fate from apoptosis to necrosis [61]. Similarly, when caspase-8-mediated extrinsic apoptosis is inhibited, cells may default to MLKL-mediated necroptosis or other inflammatory death pathways [1].
Detailed Experimental Protocols
Protocol: Evaluating Z-VAD-FMK in Noise-Induced Hearing Loss
The following workflow diagram outlines the key experimental steps from a recent in vivo study on Z-VAD-FMK efficacy:
Diagram 2: Experimental Workflow for Evaluating Z-VAD-FMK in Noise-Induced Hearing Loss. This diagram outlines the key methodological steps from a recent in vivo study demonstrating the therapeutic potential of Z-VAD-FMK.
Materials and Reagents:
- Z-VAD-FMK (commercially available from multiple suppliers) [37] [66] [62]
- Brown Norway rats (15-17 weeks old, equal sex distribution)
- Auditory brainstem response (ABR) equipment with soundproof chamber
- Octave-band noise generator (4-8 kHz, 110 dB SPL capability)
- Paraformaldehyde (4%) for tissue fixation
- EDTA solution (10%) for decalcification
- Primary antibodies for caspase-9 and IL-1β
- Immunohistochemistry supplies
Detailed Methodology:
- Pre-Exposure Baseline: Measure baseline ABR thresholds at frequencies 2, 4, 8, 16, 24, and 32 kHz under anesthesia (ketamine/xylazine cocktail) [21].
- Noise Exposure: Expose awake, unrestrained rats to 110 dB SPL octave-band noise (4-8 kHz) for 1 hour in a calibrated sound exposure system [21].
- Drug Administration: At 6 hours post-noise exposure, administer Z-VAD-FMK intraperitoneally at 3 mg/kg dose, dissolved in 10% DMSO. Include vehicle control group (10% DMSO alone) and noise-exposed untreated controls [21].
- Post-Exposure Monitoring: Conduct ABR measurements at days 1, 3, 7, 14, and 28 following noise exposure, using identical parameters to baseline measurements [21].
- Tissue Processing: On day 28, perfuse animals and collect cochleae for analysis. Fix tissues in 4% paraformaldehyde for 48 hours, then decalcify in 10% EDTA for 3 weeks [21].
- Hair Cell Quantification: Dissect cochlear whole mounts and count outer hair cells across basal, middle, and apical turns using fluorescence microscopy [21].
- Protein Analysis: Assess caspase-9 and IL-1β levels in cochlear tissues via Western blot or ELISA 24 hours post-intervention [21].
Protocol: Assessing Z-VAD-FMK in MSC Apoptosis
Experimental Design for Mesenchymal Stromal Cell Studies:
- Cell Culture: Maintain human bone marrow-derived MSCs in standard culture conditions. Confirm MSC identity through surface marker expression (CD73+, CD90+, CD105+, CD34-, CD45-) [64].
- Inflammatory Licensing: Pre-treat MSCs with TNF (20-100 ng/mL) and IFN-γ (20-50 ng/mL) for 24 hours to mimic inflammatory licensing [64].
- Death Induction: Apply apoptotic stimuli:
- FAS-mediated: Anti-FAS antibody (1-10 µg/mL) with SMAC-mimetic (500 nM)
- Intrinsic pathway: Combination BH3 mimetics (ABT-199, A-1331852, S63845 at 0.125-0.25 µM each) [64]
- Z-VAD-FMK Treatment: Apply Z-VAD-FMK (20-100 µM) concurrently with death stimuli. Include DMSO vehicle controls [64].
- Cell Death Assessment: At 3-24 hours post-treatment, analyze apoptosis by:
- Annexin V/PI staining and flow cytometry
- Caspase-3/7 activation assays
- Mitochondrial membrane potential measurements [64]
The Scientist's Toolkit: Essential Research Reagents
Table 2: Key Research Reagents for Z-VAD-FMK Studies
| Reagent/Catalog Number | Supplier Examples | Primary Function | Application Notes | |
|---|---|---|---|---|
| Z-VAD-FMK (G7231, ALX-260-020) | Promega, Enzo, ApexBT | Irreversible pan-caspase inhibitor | 20 mM stock in DMSO; working concentration 20-100 µM in vitro | [37] [62] [63] |
| Z-VAD(OMe)-FMK (27313) | BPS Bioscience | Cell-permeable caspase inhibitor | Alternative with similar specificity; inhibits caspases 1,3,4,7 | [66] |
| Q-VD-OPh | Multiple suppliers | Broad-spectrum caspase inhibitor | Lower toxicity alternative; effective at high concentrations (up to 500 µM) | [9] |
| SMAC-mimetic (Compound A) | Multiple suppliers | IAP antagonist | Synergizes with death receptor agonists (e.g., anti-FAS); use 100-500 nM | [64] |
| BH3 mimetics (ABT-199, A-1331852, S63845) | Multiple suppliers | BCL-2 family inhibitors | Induces intrinsic apoptosis; 0.125-0.25 µM each in combination | [64] |
| Recombinant FcFASL | Multiple suppliers | FAS receptor agonist | Trimeric form; more potent than anti-FAS antibody | [64] |
Discussion and Research Implications
The context-dependent outcomes of Z-VAD-FMK treatment highlight the complexity of caspase networks and their regulation across different biological systems. Several key principles emerge from the collective research:
First, cellular resistance mechanisms significantly influence Z-VAD-FMK efficacy. MSCs naturally resist extrinsic apoptosis through IAP-mediated mechanisms, requiring combined IAP antagonism for robust, Z-VAD-FMK-sensitive death [64]. Similar resistance patterns likely exist in other long-lived or stem cell populations, suggesting the need for comprehensive pathway mapping before applying caspase inhibition strategies.
Second, compensatory cell death pathways can bypass caspase inhibition. When apoptosis is effectively blocked, cells may default to necroptosis, pyroptosis, or other inflammatory death mechanisms depending on the initial stimulus and cellular context [1] [61]. This pathway plasticity represents both a challenge for therapeutic applications and an opportunity for combination targeting approaches.
Third, the timing and delivery of Z-VAD-FMK critically impact outcomes. In noise-induced hearing loss models, delayed administration (6 hours post-exposure) still provided significant protection, suggesting a window of therapeutic opportunity [21]. Similar temporal considerations likely apply in other injury models, emphasizing the importance of pharmacokinetic studies in Z-VAD-FMK application.
These findings have substantial implications for drug development targeting caspase pathways. The failure of multiple caspase inhibitors in clinical trials, attributed to inadequate efficacy or adverse safety profiles, may reflect insufficient consideration of these context-dependent factors [9]. Future research should prioritize identifying biomarkers that predict Z-VAD-FMK responsiveness across different pathological conditions and developing combination strategies that address compensatory death mechanisms.
Within caspase inhibition research, the therapeutic efficacy of the pan-caspase inhibitor Z-VAD-FMK (zVAD) is profoundly influenced by its administration timing relative to injury. This application note synthesizes experimental data to delineate the strategic advantages of pre-treatment versus post-injury protocols. The underlying thesis posits that the therapeutic window and mechanistic outcomes of zVAD are contingent on the specific pathophysiological context, influencing cell death pathways and inflammatory responses differentially based on administration timing.
Comparative Efficacy of Pre-treatment vs. Post-injury Administration
Table 1: Summary of zVAD Administration Timing and Outcomes Across Disease Models
| Disease Model | Administration Timing | Dosage & Route | Key Efficacy Outcomes | Primary Mechanism | Citation |
|---|---|---|---|---|---|
| Severe Acute Pancreatitis (SAP) | Pre-treatment | Not specified, i.p. | ↓ Lung injury score, ↓ MPO, ↓ TNF-α, ↓ IL-1β, ↓ cleaved caspase-3 | Inhibition of inflammation & apoptosis [30] | |
| Endotoxic Shock | Pre- & Post-treatment | 5, 10 mg/kg, i.p. | ↓ Mortality, alleviated disease, ↓ serum inflammatory cytokines | Induction of macrophage necroptosis; promotion of MDSCs [15] | |
| Noise-Induced Hearing Loss (NIHL) | Post-injury (6 hours) | 3 mg/kg, i.p. | ↓ Auditory threshold shifts, rescued outer hair cells, ↓ caspase-9 & IL-1β | Pan-caspase inhibition, reduced apoptosis & inflammation [21] |
Detailed Experimental Protocols
Protocol 1: Pre-treatment in Acute Pancreatitis-Associated Lung Injury
This protocol outlines pre-injury administration of zVAD to establish prophylactic protection against apoptosis-driven injury [30].
- Animal Model: Sprague-Dawley rats.
- Study Groups: Sham, SAP, SAP + zVAD-fmk.
- SAP Model Induction: Injection of 5% sodium taurocholate into the pancreatic duct.
- zVAD Administration: Administered prior to SAP model induction.
- Tissue Harvest: Animals sacrificed at 3, 6, 12, and 24 hours post-operation.
- Key Analytical Methods:
- Histopathology: H&E staining of lung tissue for injury scoring.
- ELISA: Measurement of Myeloperoxidase (MPO) activity, TNF-α, and IL-1β concentrations.
- Western Blotting: Detection of cleaved caspase-3 in lung tissues.
Protocol 2: Post-injury Treatment for Noise-Induced Hearing Loss
This protocol demonstrates the efficacy of zVAD even when administered after the injurious stimulus [21].
- Animal Model: Brown Norway rats.
- Noise Exposure: 1 hour of 110 dB continuous white-noise.
- zVAD Administration: A single intraperitoneal injection of 3 mg/kg, given 6 hours post-conclusion of noise exposure.
- Vehicle Control: 10% DMSO.
- Functional Assessment:
- Auditory Brainstem Response (ABR): Recorded pre-exposure and at days 1, 3, 7, 14, and 28 post-exposure. Thresholds, amplitudes, and latencies were measured.
- Endpoint Analyses (Day 28):
- Immunohistochemistry: Cochlear hair cell density quantification.
- Protein Analysis: Caspase-9 and IL-1β levels via immunoblotting.
Protocol 3: Modulation of Endotoxic Shock
This protocol explores timing in an inflammatory shock model, showing zVAD can be effective both before and after LPS challenge [15].
- Animal Model: Female C57BL/6 mice.
- Endotoxic Shock Induction: Lipopolysaccharide (LPS) challenge.
- zVAD Administration: Doses of 5 and 10 mg/kg, intraperitoneally. Both pre-treatment and post-treatment schedules were evaluated.
- In Vitro Cell Studies:
- Bone Marrow-Derived Macrophages (BMDMs): Pretreated with zVAD (0-80 μM) for 30 min followed by LPS (100 ng/ml) stimulation.
- Cell Viability Assay: CCK-8 reagent used to assess viability.
- Flow Cytometry: Analysis of peritoneal macrophages and myeloid-derived suppressor cells (MDSCs).
Signaling Pathways and Mechanisms
The efficacy of zVAD is determined by its interaction with key cell death and inflammatory pathways, which are differentially engaged based on administration timing.
The Scientist's Toolkit: Essential Research Reagents
Table 2: Key Reagent Solutions for zVAD-FMK Research
| Reagent / Assay | Function & Application in zVAD Research | Representative Example |
|---|---|---|
| zVAD-FMK | Broad-spectrum, irreversible caspase inhibitor. Core therapeutic/test compound in assays. | Dissolved in DMSO for in vitro use; in saline or 10% DMSO for in vivo i.p. injection [3] [21]. |
| Annexin V Probes | Detect phosphatidylserine exposure during early apoptosis. Gold standard for kinetic apoptosis analysis. | Recombinant Annexin V-FITC or -AlexaFluor 594 used in live-cell imaging [67] [68]. |
| Cell Viability Dyes | Distinguish late apoptosis/necrosis (loss of membrane integrity). Used alongside Annexin V. | YOYO-3 or DRAQ7 for compatible, non-toxic long-term live-cell imaging [67] [68]. |
| Caspase Activity Assays | Directly measure caspase enzyme activity. Confirm target engagement of zVAD. | Fluorogenic substrates like DEVD for caspases-3/7; commercial Glo caspase-3/7 assay kits [69]. |
| ELISA Kits | Quantify cytokine levels (e.g., TNF-α, IL-1β, IL-6) to assess inflammatory response modulation. | Used in endotoxic shock and pancreatitis models to demonstrate zVAD-induced cytokine reduction [30] [15]. |
| Myeloid-Derived Suppressor Cell (MDSC) Isolation Kits | Isolate CD11b+Gr-1+ immune cells for functional studies in inflammatory disease models. | Miltenyi Biotec Myeloid-Derived Suppressor Cell Isolation Kit [15]. |
The collective data underscore that the optimization of zVAD treatment timing is model-dependent. Pre-treatment strategies prove highly effective in scheduled injuries like experimental pancreatitis, primarily by prophylactically halting apoptosis and inflammation [30]. Conversely, the demonstrated success of post-injury administration in models like NIHL reveals a clinically relevant therapeutic window for intervention after damage onset, targeting ongoing apoptotic cascades [21]. The complexity is further highlighted in inflammatory diseases like endotoxic shock, where zVAD's effect is not solely dependent on timing but also on its capacity to shift cell death modalities and engage immunosuppressive cell populations [15]. These findings affirm the core thesis that a deep understanding of the dominant injury mechanisms is paramount for designing effective zVAD-based therapeutic strategies.
Within the context of caspase inhibition research, Z-VAD-FMK stands as a cornerstone reagent for investigating programmed cell death pathways. This pan-caspase inhibitor irreversibly binds to the catalytic site of caspase proteases, effectively blocking apoptosis induction and providing critical insights into cell death mechanisms [31] [70]. Its utility spans diverse research applications from fundamental caspase function studies to therapeutic intervention research in diseases characterized by dysregulated apoptosis [9]. For researchers, scientists, and drug development professionals, proper handling and application of Z-VAD-FMK is paramount for experimental reproducibility and validity. This application note provides comprehensive practical guidance on solubility, stability, and experimental implementation of Z-VAD-FMK within research protocols.
Chemical and Physical Properties
Z-VAD-FMK (Carbobenzoxy-Val-Ala-Asp-fluoromethylketone) is a cell-permeant peptide-based inhibitor designed to target the conserved active site of caspase family enzymes. The compound features an O-methylated aspartic acid at the P1 position, a modification that enhances both cellular permeability and stability compared to non-methylated analogs [70].
Table 1: Fundamental Properties of Z-VAD-FMK
| Property | Specification | Source |
|---|---|---|
| Chemical Formula | C~21~H~28~FN~3~O~7~ [71] [62] or C~22~H~30~FN~3~O~7~ [31] [63] | MedChemExpress, Enzo, Invivogen |
| Molecular Weight | 453.46 g/mol [71] [62] or 467.5 g/mol [31] [63] | MedChemExpress, Enzo, Invivogen |
| CAS Number | 161401-82-7 [71], 220644-02-0 [62], or 187389-52-2 [63] | Various suppliers |
| Purity | Typically ≥95% (HPLC/UHPLC) | Invivogen, Enzo |
Note: Variations in reported molecular formula and weight may stem from differences in salt forms or analytical methods between suppliers. Researchers should consult the specific Certificate of Analysis for their product lot.
Solubility and Storage Guidelines
Solubility Characteristics
Z-VAD-FMK exhibits high solubility in dimethyl sulfoxide (DMSO), which is the recommended solvent for preparing stock solutions. Suppliers report solubility concentrations of ≥10 mg/mL to 23.37 mg/mL in DMSO [31] [63] [62]. The compound is insoluble in ethanol and water [63], necessitating initial dissolution in DMSO before further dilution in aqueous buffers for cell culture applications.
Storage and Stability
Proper storage is critical for maintaining reagent integrity and experimental consistency.
Table 2: Stability and Storage Conditions
| Condition | Recommendation | Supporting Evidence |
|---|---|---|
| Long-Term Storage | -20°C, desiccated | Invivogen, Enzo, ApexBT |
| Reconstituted Solution | Stable up to 6 months at -20°C | Invivogen |
| Freeze-Thaw Cycles | Stable for at least 3 cycles | [23] |
| Room Temperature | Stable for at least 3 days | [23] |
| Aliquoting | Highly recommended to avoid repeated freeze-thaw cycles and moisture absorption | Invivogen, Enzo |
Preparation of Working Solutions
Stock Solution Reconstitution
- Equipment Preparation: Gather sterile microcentrifuge tubes, a calibrated micropipette, and anhydrous DMSO.
- Weighing: Accurately weigh the Z-VAD-FMK powder. Precise weighing is crucial as all subsequent concentration calculations depend on this initial mass [23].
- Dissolution: Add the required volume of anhydrous DMSO to achieve the desired stock concentration (typically 10-20 mM).
- Mixing: Gently vortex or pipette mix until the solution is clear and particulate-free.
- Aliquoting: Immediately dispense into single-use aliquots to minimize freeze-thaw cycles and exposure to moisture.
- Storage: Store aliquots at -20°C in a desiccated environment.
Working Solution Formulation
For cell culture applications, dilute the DMSO stock solution into pre-warmed serum-free medium or PBS to achieve the final working concentration. The final DMSO concentration in cell culture should generally not exceed 0.1-0.5% to avoid cytotoxicity.
Experimental Protocols
Protocol: Inhibition of Apoptosis in Cell Culture
This protocol outlines the use of Z-VAD-FMK to prevent caspase-dependent apoptosis in cell culture models, adapted from established methodologies [72] [70].
Materials and Reagents
- Z-VAD-FMK stock solution (20 mM in DMSO)
- Appropriate cell culture medium
- Apoptosis-inducing agent (e.g., chemotherapeutic drug, anti-Fas antibody)
- Cell viability assay reagents (e.g., WST-1, annexin V/PI)
Procedure
- Cell Plating: Plate cells at appropriate density (e.g., 5,000-50,000 cells/well in 96-well plates) and incubate for 24 hours to allow attachment.
- Pre-treatment: Add Z-VAD-FMK to achieve final working concentrations (typically 10-100 µM). Include vehicle control (DMSO at same dilution).
- Incubation: Incubate plates for 30 minutes to 2 hours to allow inhibitor uptake.
- Apoptosis Induction: Add apoptosis-inducing agent at predetermined concentrations.
- Incubation: Continue incubation for desired timeframe (typically 24-72 hours).
- Assessment: Quantify apoptosis using appropriate methods (e.g., annexin V/PI staining, caspase activity assays, Western blotting for cleaved caspases or PARP).
Key Considerations
- Timing: Z-VAD-FMK should be added concurrently with or prior to apoptosis induction for optimal inhibition [70].
- Concentration Optimization: Perform dose-response experiments to establish effective concentrations for specific cell types.
- Controls: Always include vehicle (DMSO) controls and untreated controls.
Protocol: Application in Tissue Transplantation Models
This protocol demonstrates the use of Z-VAD-FMK in mitigating ischemia/reperfusion injury in transplanted tissues, based on published research in ovarian tissue transplantation [72].
Materials and Reagents
- Z-VAD-FMK stock solution (20 mM in DMSO)
- Collagen matrix or appropriate tissue support scaffold
- Transplantation model system (e.g., murine model)
Procedure
- Tissue Preparation: Prepare tissue fragments (e.g., 2.5 × 2.5 × 1 mm for ovarian tissue).
- Inhibitor Incorporation: Embed tissue fragments in collagen matrix containing Z-VAD-FMK at 50 µM final concentration.
- Control Preparation: Prepare control fragments in collagen matrix without inhibitor or with vehicle only.
- Transplantation: Graft treated and control tissues into appropriate transplantation sites.
- Harvest and Analysis: Harvest tissues at predetermined endpoints (e.g., 3 days and 3 weeks post-transplantation).
- Assessment: Evaluate tissue integrity, apoptosis markers (e.g., TUNEL staining, cleaved caspase-3 immunohistochemistry), and functional outcomes.
Key Considerations
- Local Administration: Direct incorporation into support matrices provides sustained local delivery.
- Dose Optimization: Concentration may require adjustment for different tissue types.
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Research Reagent Solutions
| Reagent/Item | Function/Application | Example Usage |
|---|---|---|
| Z-VAD-FMK | Irreversible pan-caspase inhibitor; blocks apoptosis execution | Core inhibitor in apoptosis studies; used at 10-100 µM in cell culture [72] [70] |
| Anhydrous DMSO | Solvent for stock solution preparation; ensures compound stability | Preparation of 10-20 mM stock solutions [23] |
| Annexin V/Propidium Iodide | Apoptosis detection via flow cytometry | Differentiates early apoptotic (Annexin V+/PI-) from late apoptotic/necrotic (Annexin V+/PI+) cells [72] |
| PARP Antibodies | Western blot detection of caspase-mediated cleavage | Apoptosis confirmation through detection of cleaved PARP fragments [72] |
| Caspase Activity Assays | Direct measurement of caspase enzymatic activity | Validation of inhibitor efficacy in cellular extracts |
| Collagen Matrix | 3D support system for tissue/organ culture | Localized delivery of Z-VAD-FMK in transplantation models [72] |
Mechanism of Action and Pathway Integration
Z-VAD-FMK functions as an irreversible caspase inhibitor through covalent modification of the catalytic cysteine residue in the caspase active site. The fluoromethyl ketone (FMK) group serves as an electrophilic trap that forms a thioether bond with the cysteine thiolate, permanently inactivating the enzyme [73]. This inhibition prevents proteolytic processing of caspase substrates, thereby halting the apoptotic cascade.
Troubleshooting and Optimization
Common Challenges and Solutions
- Poor Inhibition Efficacy: Ensure proper storage and handling to maintain compound activity. Verify expiration date and prepare fresh stock solutions if needed.
- Cellular Toxicity: Optimize DMSO concentration to ensure final concentration remains below cytotoxic levels (typically <0.5%).
- Incomplete Protection: Consider that some cell death pathways may be caspase-independent. Combine with other inhibitors (e.g., necroptosis inhibitors) for broad-spectrum protection [72].
- Concentration Optimization: Perform dose-response curves for each new cell type or application, as effective concentrations can vary significantly.
Important Considerations for Experimental Design
- Timing of Administration: Z-VAD-FMK is most effective when administered prior to or concurrently with apoptosis induction.
- Cell Permeability: The O-methylated aspartic acid enhances cellular uptake, but permeability may vary between cell types.
- Specificity Limitations: While considered a pan-caspase inhibitor, Z-VAD-FMK may not equally inhibit all caspase family members and can have off-target effects at higher concentrations [74].
Z-VAD-FMK remains an invaluable tool for caspase research when applied with careful attention to solubility characteristics, stability requirements, and appropriate experimental protocols. Proper handling—including storage at -20°C, use of anhydrous DMSO for solubilization, and avoidance of repeated freeze-thaw cycles—ensures reagent integrity and experimental reproducibility. The protocols outlined herein provide researchers with robust methodologies for implementing this caspase inhibitor across diverse experimental systems, from cell culture to complex tissue models. Through meticulous application of these practical considerations, scientists can reliably leverage Z-VAD-FMK to advance our understanding of caspase-mediated pathways in health and disease.
Caspases are cysteine-dependent proteases that play central roles in programmed cell death and inflammation, with their activity rigorously defined by a primary specificity for cleaving after aspartic acid residues [51] [1]. Z-VAD-FMK (carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone) is a pan-caspase inhibitor that functions by irreversibly binding to the catalytic site of caspases, effectively inhibiting a broad spectrum of these enzymes [31]. However, despite its widespread use as a tool compound in research, accumulating evidence demonstrates that Z-VAD-FMK produces significant off-target effects that extend beyond caspase inhibition, particularly through the induction of alternative cell death pathways such as necroptosis [15] [9]. Recognizing and controlling for these non-caspase interactions is essential for the accurate interpretation of experimental results and for advancing therapeutic applications of caspase modulation.
Table 1: Documented Off-Target Effects of Z-VAD-FMK
| Off-Target Effect | Experimental Context | Key Mediators | Functional Outcome |
|---|---|---|---|
| Induction of necroptosis [15] | LPS-stimulated macrophages | RIPK1, RIPK3, MLKL, NO [15] | Promotes inflammatory cell death |
| Modulation of immune cell populations [15] | Murine endotoxic shock model | Myeloid-derived suppressor cells (MDSCs) [15] | Alters inflammatory cytokine production |
| Inhibition of non-caspase proteases [9] | In vitro enzyme assays | Other cysteine proteases (e.g., granzyme B) [9] | Potential disruption of additional signaling pathways |
Key Experimental Protocols for Assessing Off-Target Effects
Protocol for Differentiating Apoptosis and Necroptosis In Vitro
Purpose: To determine whether cell death occurring in the presence of Z-VAD-FMK is due to the intended inhibition of apoptosis or the off-target induction of necroptosis.
Reagents Required:
- Bone marrow-derived macrophages (BMDMs) or other relevant cell line
- Z-VAD-FMK (e.g., 20-80 µM working concentration) [15]
- Lipopolysaccharide (LPS) (e.g., 100 ng/mL) as an inflammatory stimulus [15]
- Necrostatin-1 (Nec-1, 10-100 µM), a specific RIPK1 inhibitor [15]
- Cell viability assay (e.g., CCK-8 reagent) [15]
- Nitric oxide detection assay
- Pro-inflammatory cytokine ELISA kits (e.g., for TNF-α, IL-6)
Methodology:
- Cell Preparation: Seed BMDMs at a density of 1 × 10^5 cells per well in a 96-well plate and allow to adhere overnight [15].
- Experimental Pretreatment: Pre-treat cells for 30 minutes with one of the following conditions:
- Vehicle control (DMSO)
- Z-VAD-FMK (e.g., 40 µM)
- Necrostatin-1 (e.g., 30 µM)
- Combination of Z-VAD-FMK and Necrostatin-1 [15]
- Stimulation: Challenge all groups with LPS (100 ng/mL) for 24-48 hours [15].
- Assessment:
- Viability: Measure cell viability using CCK-8 reagent according to manufacturer's instructions [15].
- Necroptosis Induction: Quantify nitric oxide production in culture supernatants as a key mediator of Z-VAD-FMK-promoted necroptosis [15].
- Inflammation Profile: Analyze levels of pro-inflammatory cytokines (TNF-α, IL-6) via ELISA.
- Interpretation: Protection by Necrostatin-1 in Z-VAD-FMK-treated cells indicates off-target necroptosis. Reduced pro-inflammatory cytokines in Z-VAD-FMK groups suggest a combined effect of caspase inhibition and necroptosis.
Protocol for Evaluating Immune Cell Population Shifts In Vivo
Purpose: To assess the off-target effects of Z-VAD-FMK on the accumulation and function of immunosuppressive cell populations in a disease model.
Reagents Required:
- C57BL/6 mice (6-8 weeks old)
- Z-VAD-FMK (e.g., 5-10 mg/kg for in vivo administration) [15]
- LPS for endotoxic shock induction
- Fluorescence-activated cell sorting (FACS) buffer and antibodies:
- CD11b-FITC, Gr-1-PE for total MDSCs
- Ly-6G-PerCP, Ly-6C-APC for granulocytic (G-MDSC) and monocytic (M-MDSC) subsets [15]
- Myeloid-Derived Suppressor Cell Isolation Kit
Methodology:
- Animal Model: Induce endotoxic shock by administering LPS to mice.
- Inhibition: Treat mice with Z-VAD-FMK or vehicle control via intraperitoneal injection either before or after LPS challenge [15].
- Cell Isolation: After 12-24 hours, harvest spleens and peritoneal cavities to isolate immune cells.
- Flow Cytometry:
- Stain single-cell suspensions with fluorochrome-conjugated antibodies against CD11b, Gr-1, Ly-6G, and Ly-6C.
- Analyze using flow cytometry to identify and quantify MDSC populations (CD11b+Gr-1+), with G-MDSCs as CD11b+Ly6G+Ly6Clow and M-MDSCs as CD11b+Ly6G−Ly6Chigh [15].
- Functional Suppression Assay:
- Co-culture purified G-MDSCs with BMDMs (e.g., 2 × 10^5 cells/well) for 12 hours.
- Stimulate with LPS (100 ng/mL) for 24 hours.
- Analyze macrophages by flow cytometry or measure cytokine production to assess the suppressive function of MDSCs [15].
Signaling Pathways and Molecular Interactions
The following diagram illustrates the complex signaling pathways through which Z-VAD-FMK exerts both its on-target caspase inhibitory effects and its off-target activities, leading to necroptosis and modulation of the immune response.
The Scientist's Toolkit: Essential Research Reagents
Table 2: Key Reagents for Studying Z-VAD-FMK Off-Target Effects
| Reagent | Function/Application | Key Considerations |
|---|---|---|
| Z-VAD-FMK [31] | Irreversible pan-caspase inhibitor; tool compound for inducing necroptosis under specific stimuli [15]. | Working concentration: ~20-80 µM for cell culture; 5-10 mg/kg for murine studies [15] [31]. Soluble in DMSO [31]. |
| Necrostatin-1 [15] | Selective RIPK1 inhibitor; used to confirm necroptosis induction as an off-target effect of Z-VAD-FMK. | Validates the specific pathway involved; typically used at 10-100 µM in vitro. |
| LPS (Lipopolysaccharide) [15] | TLR4 agonist; provides inflammatory stimulus to trigger cell death pathways in macrophages. | Common concentration: 100 ng/mL for in vitro macrophage stimulation [15]. |
| Cell Viability Assays (e.g., CCK-8) [15] | Quantifies metabolic activity to assess cell death/survival in response to Z-VAD-FMK treatment. | Distinguishes overall cytotoxicity but does not differentiate death modalities alone. |
| Antibodies for Flow Cytometry (CD11b, Gr-1, Ly-6G, Ly-6C) [15] | Identifies and quantifies MDSC populations and macrophage activation states in complex samples. | Essential for detecting Z-VAD-FMK-induced shifts in immune cell populations in vivo. |
| Myeloid-Derived Suppressor Cell Isolation Kit [15] | Isolates pure MDSC populations for functional suppression assays. | Enables direct testing of MDSC-mediated macrophage inhibition. |
The investigation of Z-VAD-FMK's off-target effects, particularly its capacity to induce necroptosis and modulate immune responses, reveals critical limitations of this pharmacological tool [15] [9]. These findings underscore the necessity of employing rigorous control strategies in experimental design. To accurately interpret results from studies utilizing Z-VAD-FMK, researchers should implement the following approaches: (1) consistently use specific pathway inhibitors like Necrostatin-1 to discriminate between on-target and off-target effects; (2) employ multiple complementary assays to fully characterize cell death modalities; and (3) monitor immune cell population changes in vivo, as these can significantly influence experimental outcomes and therapeutic efficacy [15]. Acknowledging and systematically controlling for these non-caspase interactions is fundamental to advancing our understanding of cell death mechanisms and developing effective caspase-targeted therapies.
Validation Studies and Comparative Analysis with Other Caspase-Targeting Agents
Within caspase inhibition research, particularly concerning pan-caspase inhibitors like Z-VAD-FMK, confirming functional efficacy is paramount. Functional assays move beyond merely confirming the presence of the inhibitor to demonstrating its biological activity—effectively blocking caspase-mediated processes in physiologically relevant models. For researchers investigating the Z-VAD-FMK mechanism, this validation is a crucial step that bridges biochemical binding with phenotypic outcomes in apoptosis, inflammation, and other caspase-driven pathways. The complex interplay of cell death pathways, including the recently characterized cuproptosis [75] and the unique eryptosis in erythrocytes [76], further underscores the necessity of specific, well-validated caspase inhibition tools. This document provides detailed application notes and protocols for key functional assays, enabling robust validation of caspase inhibition within a drug development and basic research framework.
A foundational understanding of caspase function is essential for designing effective validation strategies. Caspases are a family of cysteine proteases that are master regulators of apoptosis and inflammation. The historic belief of caspases as mediators of apoptosis and inflammation has rendered them as attractive targets for the treatment of several diseases including neurodegeneration, inflammation, metabolic disease, and cancer [9]. However, achieving inhibitor selectivity for individual members of this highly homologous enzyme family remains a major challenge [77]. Furthermore, emerging evidence has shown the activation of alternative caspase-independent cell death processes upon caspase inhibition, highlighting the need for careful experimental design that can distinguish between different cell death modalities [9].
Table: Core Characteristics of Caspase Inhibitors in Research
| Inhibitor Name | Primary Specificity | Key Mechanism | Common Research Applications |
|---|---|---|---|
| Z-VAD-FMK | Pan-caspase | Irreversibly binds catalytic site cysteine; prevents pro-caspase activation [12] [78]. | Broad-spectrum apoptosis inhibition; distinguishing caspase-dependent/independent death [12] [78]. |
| Q-VD-OPh | Pan-caspase | Irreversible, broad-spectrum inhibitor with reduced cellular toxicity [9]. | In vivo models and long-term cell culture where toxicity is a concern [9]. |
| Ac-DEVD-CHO | Caspase-3/7 | Reversible aldehyde inhibitor targeting the executioner caspases [9]. | Biochemical analysis of caspase-3/7 activity. |
| VX-765 (Belnacasan) | Caspase-1 | Reversible peptidomimetic inhibitor of inflammatory caspases [9]. | Investigation of inflammasome and IL-1β driven pathologies. |
Key Functional Assays for Caspase Inhibition
In Vivo Functional Validation: Auditory Brainstem Response (ABR) in Noise-Induced Hearing Loss (NIHL) Models
Background and Principle The in vivo model of noise-induced hearing loss (NIHL) provides a robust system for functionally testing the neuroprotective efficacy of caspase inhibitors like Z-VAD-FMK. Acoustic overexposure triggers a well-characterized pathogenic cascade in the cochlea, including oxidative stress, inflammation, and ultimately, cellular apoptosis of hair cells and neurons [21]. The ABR assay serves as a non-invasive, quantitative measure of auditory function, allowing for longitudinal assessment of treatment effects on hearing threshold shifts, a direct functional readout of caspase-mediated damage.
Detailed Experimental Protocol
- Animal Model and Groups: Utilize Brown Norway rats (15-17 weeks old). Assign animals to four groups: (1) unexposed control, (2) noise-exposed, (3) noise + vehicle, and (4) noise + Z-VAD-FMK. Include equal numbers of males and females [21].
- Noise Exposure Paradigm: Expose animals to octave-band noise (4–8 kHz) at 110 dB SPL for 1 hour. Animals should be kept awake and unrestrained in a calibrated soundproof chamber with even sound distribution [21].
- Drug Administration: Prepare Z-VAD-FMK by dissolving in 10% DMSO. Administer a single intraperitoneal injection at a dosage of 3 mg/kg, delivered 6 hours post-noise exposure [21].
- ABR Recording and Timeline:
- Anesthesia: Anesthetize animals using a ketamine (44 mg/kg, i.m.) and xylazine (5 mg/kg, i.m.) cocktail.
- Electrode Placement: Position subdermal needle electrodes at the vertex (reference), ventrolateral to the tested ear (active), and near the hind leg (ground).
- Stimulation and Thresholding: Present acoustic stimuli (2, 4, 8, 16, 24, and 32 kHz) starting at 90 dB SPL, decreasing in 5 dB steps. The auditory threshold is defined as the lowest intensity at which a reproducible Wave I and/or Wave IV can be visually identified.
- Suprathreshold Analysis: Record Wave I amplitude and latency at a suprathreshold level (e.g., 80 dB SPL) to assess the functional integrity of the peripheral auditory nerve.
- Assessment Schedule: Record ABRs pre-exposure (baseline) and at days 1, 3, 7, 14, and 28 post-intervention [21].
Data Interpretation and Analysis Effective caspase inhibition with Z-VAD-FMK manifests as a significant mitigation of permanent threshold shifts (PTS), particularly at low and mid-frequencies, compared to the noise-exposed and vehicle control groups. Treatment should also rescue the reduction in Wave I amplitude and the increase in Wave I latency, indicating protection of cochlear hair cells and synaptic function [21]. Protein analysis from cochlear tissues harvested 24 hours post-intervention should show reduced levels of key apoptotic markers like caspase-9 and the inflammatory cytokine IL-1β, providing molecular corroboration for the functional ABR findings [21].
Biochemical Validation: Caspase Activity Assays
Background and Principle Directly measuring the enzymatic activity of caspases in cell lysates or tissue homogenates provides a quantitative biochemical assessment of inhibitor efficacy. The Caspase-Glo 3/7 Assay is a prime example of a homogeneous, bioluminescent assay that leverages a proluminescent substrate containing the DEVD tetrapeptide sequence, which is cleaved specifically by caspase-3 and -7 [79]. The resulting luminescent signal is directly proportional to caspase activity, allowing for high-throughput screening of inhibitor potency.
Detailed Experimental Protocol
- Reagent Preparation: Thaw the Caspase-Glo 3/7 Buffer and Substrate and allow them to equilibrate to room temperature. Reconstitute the lyophilized substrate with the buffer to form the Caspase-Glo 3/7 Reagent. Protect from light [79].
- Cell Culture and Treatment:
- Seed cells (e.g., Jurkat T cells) in a white-walled multiwell plate at an optimal density (e.g., 10,000 cells/well in a 96-well format).
- Induce apoptosis by adding your chosen stimulus (e.g., anti-Fas antibody, staurosporine, bortezomib). Include a negative control (untreated cells) and a background control (cell-free medium).
- Pre-treat or co-treat cells with Z-VAD-FMK (typical working concentration range 10-100 μM) to test inhibitory efficacy [12] [78].
- Assay Execution:
- At the desired timepoint post-induction, remove the plate from the incubator and equilibrate to room temperature for approximately 15-30 minutes.
- Add an equal volume of Caspase-Glo 3/7 Reagent to each well (e.g., 100 μL reagent to 100 μL of medium containing cells in a 96-well plate).
- Mix the contents gently using a plate shaker for 30-60 seconds to ensure homogeneous lysis.
- Incubate the plate at room temperature for 1 hour to allow for signal development.
- Measure the luminescence using a plate-reading luminometer [79].
Data Interpretation and Analysis Successful caspase inhibition by Z-VAD-FMK will result in a significant decrease in luminescence signal compared to the apoptosis-induced, untreated control. The data can be expressed as relative luminescence units (RLU) or normalized as a percentage of the maximum apoptosis signal. A dose-response curve can be generated by treating cells with a titration of Z-VAD-FMK, allowing for the calculation of IC₅₀ values, which provides a quantitative metric of inhibitor potency in a specific cellular context.
Table: Quantitative Data from a Representative Caspase-Glo 3/7 Assay [79]
| Treatment Condition | Luminescence (RLU) | Interpretation |
|---|---|---|
| Untreated Jurkat Cells | Low background signal (~5,000 RLU) | Baseline, healthy cells |
| Jurkat Cells + Anti-Fas mAb | High signal (~100,000 RLU) | Robust caspase-3/7 activation |
| Jurkat Cells + Anti-Fas mAb + Z-VAD-FMK (20μM) | Signally markedly reduced (~15,000 RLU) | Effective caspase inhibition |
| Cell-Free Background | Minimal signal (~500 RLU) | Assay reagent background |
Phenotypic Validation: Cell Death and Viability Assays
Background and Principle The ultimate functional validation of a caspase inhibitor is its ability to prevent cell death and maintain cell viability. This is typically assessed using assays that measure hallmark features of apoptosis, such as phosphatidylserine (PS) externalization and membrane integrity. The Annexin V/Propidium Iodide (PI) staining assay, analyzed by flow cytometry, is a gold standard for this purpose.
Detailed Experimental Protocol
- Cell Treatment: Induce apoptosis in your chosen cell line (e.g., THP-1 or Jurkat) with an appropriate stimulus. Pre-treat cells with Z-VAD-FMK (e.g., 20-50 μM) for 30-60 minutes prior to the apoptosis inducer [12] [78].
- Staining Procedure:
- After treatment, harvest cells by gentle centrifugation and wash with cold PBS.
- Resuspend the cell pellet in a binding buffer at a density of 1-5 x 10⁵ cells/100 μL.
- Add Annexin V-FITC and Propidium Iodide (PI) to the cell suspension according to the manufacturer's instructions.
- Incubate the mixture for 15 minutes at room temperature in the dark.
- Add additional binding buffer and analyze by flow cytometry within 1 hour.
- Flow Cytometry Analysis:
- Annexin V-/PI-: Viable, healthy cells.
- Annexin V+/PI-: Early apoptotic cells (PS externalized, membrane intact).
- Annexin V+/PI+: Late apoptotic or necrotic cells.
- Annexin V-/PI+: Necrotic cells or debris.
Data Interpretation and Analysis Effective caspase inhibition with Z-VAD-FMK will result in a substantial decrease in the percentage of cells in the early apoptotic (Annexin V+/PI-) and late apoptotic (Annexin V+/PI+) quadrants, with a corresponding increase in the viable (Annexin V-/PI-) population. This confirms that Z-VAD-FMK functionally blocks the apoptotic cascade. It is critical to note that Z-VAD-FMK does not inhibit caspase-independent forms of cell death, such as ferroptosis or necroptosis, which should be ruled out with specific inhibitors if observed [12].
The Scientist's Toolkit: Essential Reagents and Materials
Table: Key Research Reagent Solutions for Caspase Inhibition Studies
| Reagent/Material | Function/Description | Example Product/Specification |
|---|---|---|
| Z-VAD-FMK | Cell-permeant, irreversible pan-caspase inhibitor. Typically supplied as a solution in DMSO [37]. | Promega Catalog #G7231 (discontinued, many alternatives available from other vendors like APExBIO) [37]. |
| Caspase-Glo 3/7 Assay System | Homogeneous, bioluminescent assay for measuring caspase-3/7 activity. "Add-mix-measure" format [79]. | Promega Catalog #G8091, etc. Includes buffer and lyophilized substrate [79]. |
| Annexin V/Propidium Iodide Kit | Flow cytometry-based assay for detecting phosphatidylserine externalization (apoptosis) and membrane integrity. | Available from multiple vendors (e.g., BioLegend, Thermo Fisher). |
| Cell Culture Plates | Multiwell plates for cell-based assays. | White-walled, clear-bottom plates for luminescence assays; standard plates for flow cytometry. |
| MyGlo Reagent Reader | Luminometer for measuring bioluminescent signals from assays like Caspase-Glo 3/7 [79]. | Promega Catalog #MG1010 [79]. |
Integrated Data Analysis and Pathway Mapping
Successful functional validation of caspase inhibition requires correlating data from multiple assays. For instance, in the NIHL model, the functional ABR recovery should be supported by histological evidence of rescued outer hair cells and a molecular reduction in caspase-9 and IL-1β levels [21]. This integrated approach confirms that the inhibitor is engaging its target, blocking the downstream apoptotic pathway, and ultimately preserving tissue structure and function.
Robust functional validation is the cornerstone of credible research into caspase inhibition. The synergistic application of in vivo functional tests like ABR, biochemical activity assays like Caspase-Glo 3/7, and phenotypic flow cytometry assays provides a multi-faceted and compelling confirmation of the efficacy of Z-VAD-FMK and related compounds. These protocols, grounded in current literature and employing standardized reagents, provide a rigorous framework for researchers and drug development professionals to validate the functional impact of caspase inhibition within the complex landscape of cell death and inflammation.
Caspases are cysteine-dependent proteases that serve as master regulators of programmed cell death (PCD), playing crucial roles in apoptosis, pyroptosis, and inflammation [1]. The dysregulation of caspase-mediated pathways is implicated in various diseases, including cancer, neurodegenerative disorders, inflammatory conditions, and liver diseases [9] [1]. Synthetic caspase inhibitors have emerged as valuable therapeutic tools and research reagents to modulate these pathways. Among the most prominent are Z-VAD-FMK, a first-generation pan-caspase inhibitor; Q-VD-OPh, an advanced broad-spectrum inhibitor with reduced toxicity; and Emricasan (IDN-6556), a peptidomimetic inhibitor that has progressed to clinical trials. This application note provides a comparative analysis of these three inhibitors, detailing their mechanisms, efficacy, and applications in research and therapeutic contexts, with structured protocols for experimental use.
Comparative Profiling of Caspase Inhibitors
The table below summarizes the key characteristics of Z-VAD-FMK, Q-VD-OPh, and Emricasan, highlighting their pharmacological profiles and experimental applications.
Table 1: Comprehensive Comparison of Synthetic Caspase Inhibitors
| Feature | Z-VAD-FMK | Q-VD-OPh | Emricasan (IDN-6556) |
|---|---|---|---|
| Chemical Class | Peptide-based, fluoromethyl ketone | Peptide-based, phenoxy | Peptidomimetic |
| Inhibition Mechanism | Irreversible, broad-spectrum | Irreversible, broad-spectrum | Irreversible, broad-spectrum |
| Cellular Permeability | Good | Excellent (brain permeable) | Good |
| Key IC₅₀ Values | Caspase-1: 0.4 nM (CrmA-sensitive)Caspase-3: 2.3 nMCaspase-8: 0.6 nM | Caspase-1: 50 nMCaspase-3: 25 nMCaspase-8: 100 nMCaspase-9: 430 nM | Pan-caspase inhibitor; specific IC₅₀ values not fully detailed in sources |
| Toxicity Concerns | Cytotoxic at high doses; can induce necroptosis in macrophages [7] | Minimal toxicity even at high concentrations (up to 500-1000 µM) [80] [81] | Well-tolerated in clinical trials; some liver toxicity concerns at high doses [9] |
| Primary Research Applications | In vitro apoptosis inhibition; studying necroptosis and inflammatory models [7] | High-fidelity apoptosis inhibition in sensitive models (e.g., neurons); in vivo disease models [82] [80] | Animal models of liver disease (e.g., BDL); clinical trials for liver fibrosis and FECD [83] [84] |
| Therapeutic Status | Research tool only | Research tool only | Advanced clinical trials for liver diseases and Fuchs Endothelial Corneal Dystrophy (FECD) [84] [85] |
Detailed Experimental Protocols
Protocol for Evaluating Caspase Inhibition in an Endotoxic Shock Model Using Z-VAD-FMK
This protocol is adapted from studies investigating the role of Z-VAD-FMK in alleviating endotoxic shock [7].
Application: To study the anti-inflammatory effects of caspase inhibition and its potential to induce necroptosis in a lipopolysaccharide (LPS)-induced shock model.
Reagents and Materials:
- C57BL/6 mice (6-8 weeks old)
- Z-VAD-FMK (e.g., from Beyotime Biotechnology)
- Lipopolysaccharide (LPS)
- Thioglycollate medium
- Cell culture reagents for BMDMs: DMEM, GM-CSF (10 ng/ml)
- Flow cytometry equipment and antibodies for MDSC analysis (CD11b, Ly6G, Ly6C)
- ELISA kits for cytokines (TNF-α, IL-6)
Procedure:
- Animal Pre-treatment: Administer Z-VAD-FMK intraperitoneally (i.p.) at doses of 5, 10, or 20 µg per gram of body weight. A vehicle control (saline) must be included.
- Disease Induction: After 2 hours, induce endotoxic shock by i.p. injection of LPS at 10-50 µg/g body weight.
- Sample Collection:
- Collect serum samples at 6 hours post-LPS challenge for cytokine analysis.
- Harvest peritoneal cells at 6 and 12 hours for viability and cell death analysis (e.g., propidium iodide staining).
- Collect livers, lungs, and spleens at 12 hours for histopathological examination.
- In Vitro Validation:
- Isolate and culture Bone Marrow-Derived Macrophages (BMDMs).
- Pre-treat BMDMs with Z-VAD-FMK (0, 20, 40, 80 µM) for 30 minutes, then stimulate with LPS (100 ng/ml).
- Assess cell viability (CCK-8 assay), necroptosis markers, and cytokine secretion.
Key Analysis: Monitor mouse survival, serum cytokine levels, percentage of peritoneal macrophages, and accumulation of Myeloid-Derived Suppressor Cells (MDSCs). In vitro, correlate Z-VAD-FMK concentration with the shift from apoptosis to necroptosis.
Protocol for Assessing Anti-apoptotic Efficacy and Reduced Toxicity of Q-VD-OPh
This protocol leverages the superior safety profile of Q-VD-OPh for sensitive in vitro and in vivo applications [82] [80] [81].
Application: To inhibit apoptosis efficiently in contexts where traditional inhibitors like Z-VAD-FMK exhibit toxicity, such as in neuronal cultures or long-term in vivo studies.
Reagents and Materials:
- Q-VD-OPh (commercially available, e.g., CAS 1135695-98-5)
- Appropriate cell lines (e.g., Jurkat T-cells, primary neurons) or animal models (e.g., rat neonatal stroke model)
- Apoptosis inducers (e.g., etoposide, UV radiation, neurotoxins)
- Apoptosis detection kits (Annexin V/PI, TUNEL assay)
- Cell viability assay (e.g., MTT, CCK-8)
Procedure:
- In Vitro Cell Treatment:
- Prepare a stock solution of Q-VD-OPh in DMSO. For aqueous media, note that its aspartyl residue is not O-methylated to reduce hydrophobicity [82].
- Pre-treat cells with Q-VD-OPH (typical working concentration range 1-100 µM) for 1-2 hours before applying an apoptotic stimulus.
- Co-incubate cells with the stimulus and Q-VD-OPh for 12-48 hours.
- Viability and Apoptosis Assessment:
- Quantify apoptosis using flow cytometry (Annexin V/PI) or a TUNEL assay.
- Measure overall cell viability using a colorimetric assay (e.g., CCK-8).
- In Vivo Administration:
- In animal models of stroke or spinal cord injury, administer Q-VD-OPh intraperitoneally. Doses used in literature range from 1-10 mg/kg.
- Assess functional recovery and histopathological damage at predetermined endpoints.
Key Analysis: Compare the percentage of apoptotic cells and overall viability in Q-VD-OPh treated groups versus groups treated with other inhibitors (e.g., Z-VAD-FMK) and untreated controls. Its effectiveness is demonstrated by robust apoptosis inhibition without a loss in viability at high concentrations.
Protocol for Investigating Therapeutic Potential of Emricasan in Disease Models
This protocol outlines the use of Emricasan in preclinical models of liver disease and Fuchs Endothelial Corneal Dystrophy (FECD) [83] [84].
Application: To evaluate the anti-fibrotic and cytoprotective effects of pan-caspase inhibition in chronic disease models.
Reagents and Materials:
- Emricasan (IDN-6556)
- Bile Duct Ligation (BDL) mouse model or Col8a2Q455K/Q455K mouse model (for FECD)
- Control animals (sham-operated)
- ALT assay kit
- Reagents for histology (Sirius Red, TUNEL, α-SMA antibody)
- Flow cytometry reagents for Microparticle (MP) analysis (Annexin V)
Procedure: A. Liver Disease Model (BDL):
- Surgery and Dosing: Subject mice to BDL or sham surgery. Begin daily intraperitoneal injections of Emricasan (10 mg/kg/day) or placebo immediately post-operation.
- Long-term Monitoring: Continue treatment for 20 days, monitoring survival rates.
- Endpoint Analysis:
- Measure portal pressure via catheterization of the ileocolic vein.
- Collect blood and liver tissue.
- Analyze serum for ALT levels and circulating keratin-18+ microparticles (via flow cytometry).
- Assess liver fibrosis (Sirius Red staining) and cell death (TUNEL assay).
B. Fuchs Endothelial Corneal Dystrophy (FECD) Model:
- Treatment: Administer 0.1% Emricasan eye drops twice daily to Col8a2Q455K/Q455K mice from 8 to 28 weeks of age. Use vehicle-treated mice as controls.
- Endpoint Analysis:
- Evaluate corneal endothelium via contact specular microscopy (cell density, hexagonality).
- Analyze corneas for apoptosis (TUNEL) and extracellular matrix (ECM) accumulation.
Key Analysis: In liver models, the primary outcomes are reduced portal pressure, decreased circulating MPs, and improved survival. In FECD models, key metrics are increased endothelial cell density and reduced guttae area.
Signaling Pathways and Molecular Mechanisms
The following diagram illustrates the central role of caspase-8 as a molecular switch between cell death pathways and the points of inhibition for Z-VAD-FMK, Q-VD-OPh, and Emricasan.
Diagram Title: Caspase-8 as a Molecular Switch in Cell Death Pathways
This diagram delineates the critical role of caspase-8, which, when active, promotes apoptosis by activating executioner caspases-3/7 while simultaneously inhibiting necroptosis by cleaving RIPK1 [1]. Pan-caspase inhibitors like Z-VAD-FMK, Q-VD-OPh, and Emricasan primarily block the activation of executioner caspases. However, inhibition of caspase-8 by Z-VAD-FMK can, in certain inflammatory contexts (e.g., LPS-activated macrophages), disinhibit the necroptotic pathway, leading to RIPK1/MLKL-mediated necroptosis [7]. Q-VD-OPh and Emricasan are more effective and less toxic inhibitors of the apoptotic pathway.
The Scientist's Toolkit: Essential Research Reagents
The table below catalogues key reagents and their applications for studying caspase inhibition and its therapeutic effects.
Table 2: Essential Research Reagents for Caspase Inhibition Studies
| Reagent / Assay | Primary Function | Application Context |
|---|---|---|
| Z-VAD-FMK | Irreversible, pan-caspase inhibitor. | Studying caspase function in vitro; can induce necroptosis in inflammatory models (e.g., endotoxic shock) [7]. |
| Q-VD-OPh | Highly potent, broad-spectrum caspase inhibitor with minimal cytotoxicity. | Superior choice for sensitive apoptosis inhibition in neurons and long-term in vivo studies [82] [80] [81]. |
| Emricasan (IDN-6556) | Orally active, irreversible pan-caspase inhibitor. | Preclinical and clinical research for caspase-driven pathologies like liver fibrosis, PHT, and FECD [83] [84] [85]. |
| Annexin V / PI Staining | Distinguishes apoptotic (Annexin V+/PI-) and necrotic (Annexin V+/PI+) cells. | Quantitative assessment of cell death mode by flow cytometry. |
| TUNEL Assay | Detects DNA fragmentation, a hallmark of late-stage apoptosis. | Histological identification of apoptotic cells in tissue sections (e.g., liver, cornea) [83] [84]. |
| Caspase Activity Assays | Fluorometric or colorimetric detection of caspase enzyme activity. | Mechanistic confirmation of inhibitor efficacy on specific caspases (e.g., Caspase-3/7). |
| Sirius Red Staining | Binds to collagen fibers, highlighting fibrotic areas. | Quantification of collagen deposition and fibrosis in liver tissue [83]. |
| Flow Cytometry for Microparticles | Detects and quantifies phosphatidylserine-positive microparticles. | Assessing levels of caspase-derived circulating microparticles in disease models [83]. |
Z-VAD-FMK, Q-VD-OPh, and Emricasan represent distinct generations of caspase inhibitors with varying profiles and applications. Z-VAD-FMK remains a useful research tool but its potential for off-target effects and induction of necroptosis necessitates careful interpretation of results. Q-VD-OPh offers a superior alternative for most research applications requiring high-fidelity apoptosis inhibition with minimal toxicity. Emricasan stands out as the most therapeutically advanced candidate, showing promise in clinical trials for liver diseases and FECD by targeting apoptosis and pathological processes like ECM accumulation. The choice of inhibitor must be guided by the specific research question, model system, and the potential for compensatory cell death pathways.
Caspases are an evolutionarily conserved family of cysteine-dependent proteases that serve as crucial mediators of programmed cell death (PCD) and inflammation [1] [9]. These enzymes cleave their substrates at specific aspartic acid residues and are synthesized as catalytically inactive procaspases that require activation through trans-, recruitment-, or auto-activation mechanisms [9]. Caspases are broadly classified based on their primary functions: apoptotic initiator caspases (caspases-2, -8, -9, -10), apoptotic executioner caspases (caspases-3, -6, -7), and inflammatory caspases (caspases-1, -4, -5, -11, -12) [1] [9]. Dysregulation of caspase-mediated processes is implicated in various pathological conditions, including inflammatory diseases, neurological disorders, metabolic diseases, and cancer, making them attractive therapeutic targets [1] [9].
The development of caspase inhibitors represents a promising strategy for modulating uncontrolled cell death and inflammation in human diseases. Two major classes of natural caspase inhibitors have been identified: viral-encoded inhibitors (CrmA, p35) and cellular inhibitors (IAP proteins) [9]. These natural inhibitors provide a evolutionary blueprint for designing synthetic caspase inhibitors such as zVAD-FMK, a pan-caspase inhibitor widely used in research settings [7] [20] [21]. This application note provides a comprehensive comparison between natural and synthetic caspase inhibitors, along with detailed protocols for studying their mechanisms and applications in drug development.
Natural Caspase Inhibitors: Mechanisms and Characteristics
Viral Caspase Inhibitors
Viruses have evolved sophisticated mechanisms to evade host immune responses by encoding caspase inhibitors that protect against host-induced apoptosis [9].
Table 1: Characteristics of Viral Caspase Inhibitors
| Inhibitor | Source | Mechanism of Action | Caspase Specificity | Additional Targets |
|---|---|---|---|---|
| CrmA (Cytokine response modifier A) | Cowpox virus | Serine protease inhibitor (serpin) family; irreversibly binds and inhibits caspase active sites | Caspases-1, -8, -10 [9] | Granzyme B, cytotoxic T cell serine protease [9] |
| p35 | Baculovirus | Substrate inhibitor; forms stable complex with caspases | Multiple mammalian caspases (except caspase-9) [9] | CED-3 in C. elegans [9] |
| p49 | Baculovirus | Substrate inhibitor; prevents apoptosis in vivo | p35-insensitive initiator caspases [9] | - |
CrmA was the first identified caspase inhibitor and serves as a potent suppressor of inflammation by inhibiting caspase-1 (IL-1β converting enzyme), thereby preventing the production of mature IL-1β and interferon γ [9]. The p35 family of viral inhibitors exhibits broad-spectrum caspase inhibition through a unique substrate-mimic mechanism, forming stable complexes with multiple caspases to prevent apoptosis in infected cells [9].
Cellular Caspase Inhibitors: IAP Proteins
Inhibitor of Apoptosis (IAP) proteins constitute a family of endogenous cellular caspase regulators that play crucial roles in maintaining cellular homeostasis.
Table 2: Human IAP Family Members and Their Functions
| IAP Family Member | Caspase Targets | Mechanism of Action | Biological Functions |
|---|---|---|---|
| XIAP (X-linked IAP) | Caspases-3, -7, -9 [9] | Direct binding and inhibition via BIR domains [9] | Primary endogenous caspase inhibitor; regulates apoptosis execution |
| cIAP1 & cIAP2 | Caspases-3, -7 [9] | Direct binding and inhibition [9] | Regulate cell survival, NF-κB signaling pathway |
| Survivin | Caspase-9 [1] | Mitotic spindle association; inhibits mitochondrial apoptosis pathway [1] | Cell cycle regulation; overexpressed in cancers |
| Livin (ML-IAP) | Caspases-3, -7, -9 | BIR domain-mediated inhibition | Apoptosis regulation in development and cancer |
| NAIP | Caspase-9 | NLR family member; inhibits apoptosome formation | Neuronal apoptosis inhibition; innate immunity |
IAP proteins function through their characteristic Baculovirus IAP Repeat (BIR) domains, which facilitate direct interaction with caspase active sites [9]. Among IAP family members, XIAP represents the most potent and best-characterized endogenous caspase inhibitor, capable of directly binding and inhibiting both initiator (caspase-9) and executioner (caspases-3, -7) caspases [9].
Synthetic Caspase Inhibitors: Benchmarking Against Natural Templates
Peptide-Based Caspase Inhibitors
Synthetic caspase inhibitors were developed based on the substrate recognition patterns of natural caspases, incorporating peptide sequences that mimic natural caspase substrates [9].
Table 3: Synthetic Caspase Inhibitors and Their Applications
| Inhibitor | Type | Caspase Specificity | Research Applications | Clinical Status |
|---|---|---|---|---|
| zVAD-FMK | Irreversible pan-caspase inhibitor [7] [20] [21] | Broad-spectrum [21] [86] | Endotoxic shock models [7], noise-induced hearing loss [21], T cell proliferation studies [86] | Research use only [9] |
| Q-VD-OPh | Irreversible broad-spectrum inhibitor [9] | Broad-spectrum with reduced toxicity [9] | Neurodegeneration models, viral infection studies [9] | Preclinical development [9] |
| IDN-6556 (Emricasan) | Irreversible pan-caspase inhibitor [9] | Caspases-3, -8, -9 [9] | Liver disease models [9] | Clinical trials terminated [9] |
| VX-740 (Pralnacasan) | Peptidomimetic inhibitor [9] | Caspase-1 selective [9] | Rheumatoid arthritis, osteoarthritis models [9] | Clinical trials terminated (liver toxicity) [9] |
| VX-765 (Belnacasan) | Peptidomimetic inhibitor [9] | Caspase-1 selective [9] | Inflammatory disease models [9] | Clinical trials terminated (liver toxicity) [9] |
zVAD-FMK (benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone) represents the prototypical pan-caspase inhibitor widely used in research settings. Its structure comprises a peptide sequence (VAD) based on the recognition motif of caspase substrates, a benzyloxycarbonyl (z) group that enhances cell permeability, and a fluoromethylketone (FMK) group that irreversibly binds the catalytic cysteine residue in caspase active sites [86]. While effective in research applications, zVAD-FMK demonstrates significant off-target effects, including inhibition of cathepsin B, peptide:N-glycanase (PNGase), and picornaviral 2A proteinases, which complicate the interpretation of experimental results [86].
Comparative Analysis of Natural and Synthetic Inhibitors
Table 4: Benchmarking Natural and Synthetic Caspase Inhibitors
| Parameter | Natural Inhibitors (CrmA/IAPs) | Synthetic Inhibitors (zVAD-FMK) |
|---|---|---|
| Specificity | High specificity for particular caspase subsets [9] | Broad-spectrum with significant off-target effects [86] |
| Mechanism | Physiological regulation; protein-protein interactions [9] | Covalent modification of active site cysteine [86] |
| Toxicity | Naturally optimized for biological compatibility [9] | Dose-dependent toxicity in vivo [9] |
| Stability | Regulated by cellular degradation pathways [9] | Variable metabolic stability [9] |
| Therapeutic Potential | Limited by delivery challenges [9] | Limited by toxicity and specificity issues [9] |
| Research Utility | Elucidating physiological caspase regulation [9] | Acute caspase inhibition in experimental models [7] [20] [21] |
Natural caspase inhibitors exhibit exquisite specificity refined through evolutionary processes, while synthetic inhibitors like zVAD-FMK offer the advantage of broad-spectrum activity at the expense of increased off-target effects [9] [86]. The synthetic inhibitor Q-VD-OPh represents an improvement over zVAD-FMK, demonstrating enhanced efficacy, permeability, and reduced toxicity in vitro even at high concentrations (500-1000 µM) [9].
Experimental Protocols for Caspase Inhibition Studies
Protocol 1: Evaluating zVAD-FMK Efficacy in Endotoxic Shock Models
Background: This protocol outlines the methodology for assessing the protective effects of zVAD-FMK in lipopolysaccharide (LPS)-induced endotoxic shock, adapted from [7].
Materials:
- zVAD-FMK (commercially available from suppliers such as Beyotime Biotechnology [7])
- Lipopolysaccharide (LPS)
- C57BL/6 mice (6-8 weeks old)
- Cell culture reagents: DMEM, FBS, penicillin-streptomycin
- Cytokine measurement kits (ELISA) for TNF-α, IL-6, IL-12
- Flow cytometry equipment and antibodies for immune cell analysis
Procedure:
- Animal Pre-treatment: Administer zVAD-FMK intraperitoneally at doses ranging from 5-20 μg/g body weight 2 hours before LPS challenge [7].
- Endotoxic Shock Induction: Induce endotoxic shock by intraperitoneal injection of LPS at 10 μg/g body weight [7].
- Sample Collection: Collect serum samples 6 hours post-LPS challenge for cytokine analysis. Harvest organs (livers, lungs, spleens) 12 hours post-challenge for histopathological examination [7].
- Peritoneal Cell Analysis: Collect peritoneal cells at 6 and 12 hours for propidium iodide (PI) staining and flow cytometric analysis of cell death [7].
- Cytokine Measurement: Quantify serum levels of pro-inflammatory cytokines (TNF-α, IL-6, IL-12) using commercial ELISA kits [7].
- Histopathological Assessment: Process organ tissues for H&E staining and evaluate inflammatory infiltration and tissue damage by microscopy [7].
- Statistical Analysis: Compare survival rates, cytokine levels, and tissue damage between zVAD-FMK-treated and control groups using appropriate statistical tests (e.g., log-rank test for survival, ANOVA for cytokine levels) [7].
Expected Results: zVAD-FMK pre-treatment significantly reduces mortality in LPS-challenged mice, decreases serum pro-inflammatory cytokine concentrations, and attenuates end-organ damage, particularly in liver and lung tissues [7].
Protocol 2: Assessing Caspase Inhibition in Noise-Induced Hearing Loss Models
Background: This protocol describes the evaluation of zVAD-FMK protective effects in acoustic trauma models, based on [21].
Materials:
- zVAD-FMK (commercially available from TOCRIS R&D Systems, Cat #2163 [21])
- Brown Norway rats (15-17 weeks old)
- Auditory brainstem response (ABR) equipment
- Soundproof chamber with calibrated speakers
- Cochlear dissection tools
- Immunohistochemistry reagents: paraformaldehyde, EDTA, Triton-X, blocking serum
Procedure:
- Experimental Groups: Randomly assign animals to four groups: (1) unexposed controls, (2) noise-exposed, (3) noise + vehicle, (4) noise + zVAD-FMK (3 mg/kg) [21].
- Noise Exposure: Expose animals to continuous white noise (110 dBA, 1 hour) in a soundproof chamber with calibrated speakers [21].
- Drug Administration: Administer zVAD-FMK (3 mg/kg) intraperitoneally 6 hours after noise exposure [21].
- Auditory Function Testing: Measure auditory brainstem responses (ABR) before exposure and at days 1, 3, 7, 14, and 28 post-exposure using frequencies of 2, 4, 8, 16, 24, and 32 kHz [21].
- Tissue Collection: Euthanize animals 28 days post-intervention and harvest cochleae for histological analysis [21].
- Cochlear Processing: Fix cochleae in 4% paraformaldehyde for 2 days, decalcify in 10% EDTA for 3 weeks, and prepare for immunohistochemistry [21].
- Hair Cell Quantification: Stain cochlear tissues with hair cell markers and count surviving inner and outer hair cells along the cochlear length [21].
- Protein Analysis: Assess caspase-9 and IL-1β levels in cochlear tissues by Western blotting 24 hours post-intervention [21].
Expected Results: zVAD-FMK treatment partially mitigates noise-induced auditory threshold shifts, reduces hair cell loss, and decreases caspase-9 and IL-1β expression in cochlear tissues [21].
Protocol 3: Evaluating T Cell Proliferation Suppression by Caspase Inhibitors
Background: This protocol details the assessment of caspase inhibitor effects on human T cell proliferation, adapted from [86].
Materials:
- zVAD-FMK, z-IETD-FMK (commercially available from ICN [86])
- Human peripheral blood samples from healthy volunteers
- Lymphoprep for PBMC isolation
- RPMI 1640 medium with 10% FCS
- Mitogens: PHA, anti-CD3 (OKT3 mAb), anti-CD28 mAb
- [3H]-thymidine or CFSE for proliferation assays
- Flow cytometry antibodies: FITC-anti-CD25, RPE-anti-CD69
Procedure:
- PBMC Isolation: Isolate peripheral blood mononuclear cells (PBMCs) from heparinized blood using density gradient centrifugation with Lymphoprep [86].
- T Cell Purification: Purify CD4+ and CD8+ T cells using anti-CD4 and anti-CD8 mAb-conjugated MACS beads [86].
- Cell Culture: Culture PBMCs or purified T cells (1×10^6 cells/ml) in RPMI 1640 with 10% FCS [86].
- T Cell Activation: Stimulate cells with PHA (5 μg/ml) or co-stimulate with plate-bound anti-CD3 (5 μg/ml) and anti-CD28 (2.5 μg/ml) [86].
- Caspase Inhibitor Treatment: Treat cells with zVAD-FMK or z-IETD-FMK at non-toxic concentrations (determined by preliminary viability assays) [86].
- Proliferation Assay: Assess T cell proliferation using [3H]-thymidine incorporation or CFSE dilution by flow cytometry [86].
- Surface Marker Analysis: Analyze activation markers (CD25, CD69) by flow cytometry after 24-48 hours of stimulation [86].
- Cytokine Measurement: Quantify IL-2 and IFN-γ secretion by ELISA [86].
- Caspase Activation Assessment: Analyze processing of caspase-8 and caspase-3 by Western blotting [86].
Expected Results: Both zVAD-FMK and z-IETD-FMK suppress mitogen-induced T cell proliferation and CD25 expression without inhibiting caspase-8 and caspase-3 processing during T cell activation [86].
The Scientist's Toolkit: Essential Research Reagents
Table 5: Key Research Reagents for Caspase Inhibition Studies
| Reagent/Category | Specific Examples | Function/Application | Research Context |
|---|---|---|---|
| Pan-Caspase Inhibitors | zVAD-FMK [7] [20] [21], Q-VD-OPh [9] | Broad-spectrum caspase inhibition; apoptosis blockade | General apoptosis research; inflammatory models [7] [21] |
| Selective Caspase Inhibitors | z-IETD-FMK (caspase-8) [86], Ac-DEVD-CHO (caspase-3) [9] | Specific caspase subset inhibition | Pathway-specific studies; reducing off-target effects [86] |
| Natural Inhibitor Tools | Recombinant CrmA [9], XIAP expression constructs [9] | Physiological caspase regulation studies | Mechanistic studies; benchmarking synthetic inhibitors [9] |
| Activity Assays | Fluorogenic caspase substrates [9], Western blot for cleaved caspases [86] | Caspase activation quantification | Efficacy assessment of inhibitors [86] |
| Cell Death Detection | Propidium iodide [7], Annexin V [86], TMRE [86] | Apoptosis/necroptosis quantification | Mode-of-cell-death analysis [7] |
| Animal Models | LPS-induced endotoxic shock [7], Noise-induced hearing loss [21] | Pathophysiological relevance testing | In vivo efficacy and toxicity evaluation [7] [21] |
Caspase Signaling Pathways and Inhibitor Mechanisms
Caspase Signaling Pathways and Inhibitor Mechanisms. This diagram illustrates the hierarchical organization of caspase activation pathways and the specific points of inhibition by natural (viral CrmA and cellular IAPs) and synthetic (zVAD-FMK) inhibitors. Initiator caspases (yellow) activate effector caspases (red), which execute cell death programs. Natural inhibitors (green) demonstrate targeted specificity, while synthetic pan-inhibitors (blue) provide broad-spectrum caspase blockade.
Experimental Workflow for Caspase Inhibitor Evaluation
Experimental Workflow for Caspase Inhibitor Evaluation. This workflow outlines a systematic approach for evaluating caspase inhibitors, from initial selection through comprehensive assessment of efficacy and mechanisms. The sequential steps ensure standardized evaluation across different inhibitor classes and model systems, facilitating direct comparison between natural and synthetic compounds.
Natural caspase inhibitors such as viral CrmA and cellular IAP proteins provide valuable templates for understanding physiological caspase regulation and designing therapeutic interventions [9]. While synthetic inhibitors like zVAD-FMK offer research utility and demonstrate efficacy across diverse disease models [7] [20] [21], their clinical translation has been hampered by toxicity, off-target effects, and inadequate specificity [9]. Future development of caspase-targeting therapeutics should incorporate insights from natural inhibitor mechanisms, particularly their exquisite specificity and evolutionary optimization. Emerging strategies include developing dual-specificity inhibitors, targeting caspase regulatory complexes rather than individual enzymes, and employing nanomedicine approaches for targeted delivery to enhance therapeutic efficacy while minimizing systemic toxicity [87]. The continued benchmarking of synthetic inhibitors against natural templates remains essential for advancing caspase-targeted therapies from research tools to clinical reality.
Caspases, an evolutionarily conserved family of cysteine-dependent proteases, are master regulators of programmed cell death (apoptosis) and inflammation [9] [1]. The historic belief of caspases as mere mediators of apoptosis and inflammation has rendered them attractive therapeutic targets for numerous diseases, including neurodegeneration, inflammatory conditions, and cancer [9] [88]. For decades, research and therapeutic development have focused on designing synthetic caspase inhibitors.
A groundbreaking discovery has revealed that several Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)—among the most commonly used drugs worldwide—inhibit caspases at physiologic concentrations [89]. This identifies caspases as a novel pharmacological target for NSAIDs and suggests a COX-independent anti-inflammatory mechanism [89] [90]. This application note details this unexpected crossover, its experimental validation, and its implications for research and drug development, framing it within the broader context of caspase inhibition research where tools like the pan-caspase inhibitor Z-VAD-FMK are pivotal.
The Dual Role of Caspases in Cell Death and Inflammation
Caspases are classified based on their primary functions in apoptosis and inflammation. Table 1 summarizes the key mammalian caspases and their roles.
Table 1: Key Mammalian Caspases and Their Primary Functions
| Caspase | Classification | Primary Functions and Notes |
|---|---|---|
| Caspase-1 | Inflammatory | Processes pro-inflammatory cytokines IL-1β and IL-18; activated by inflammasomes [31] [88]. |
| Caspase-2 | Apoptotic Initiator | Involved in intrinsic apoptosis triggered by stress signals [1]. |
| Caspase-3, -6, -7 | Apoptotic Executioner | Execute apoptosis by cleaving key cellular substrates like PARP [9] [1]. |
| Caspase-4, -5, -11 | Inflammatory | Mediate pyroptosis by cleaving Gasdermin D; sense intracellular LPS [89] [1]. |
| Caspase-8, -10 | Apoptotic Initiator | Initiate extrinsic apoptosis; act as molecular switches between cell death pathways [9] [77]. |
| Caspase-9 | Apoptotic Initiator | Initiates intrinsic apoptosis via the apoptosome complex [9] [1]. |
| Caspase-12 | Inflammatory | Associated with endolasmic reticulum stress-induced apoptosis [1]. |
The following diagram illustrates the complex signaling pathways of caspase-mediated programmed cell death.
NSAIDs as Unexpected Caspase Inhibitors: Evidence and Mechanisms
Key Discovery from High-Throughput Screening
The initial discovery that NSAIDs can inhibit caspases came from a high-throughput screen of the 1,280-compound Prestwick Chemical Library (comprised of FDA-approved, bioavailable drugs) to identify inhibitors of caspase-4, an innate immune receptor that directly binds LPS [89].
Strikingly, NSAIDs constituted half of the hits and eight of the top ten most potent inhibitors, reducing caspase-4 catalytic activity to less than 25% at a concentration of 33 μM. Hits were structurally diverse and not limited to a single NSAID class [89].
Table 2: Selected NSAID Hits from Caspase-4 High-Throughput Screen
| NSAID Name | Therapeutic Category | Remaining Caspase-4 Activity |
|---|---|---|
| Fenbufen | NSAID | 3.71% |
| Ketorolac Tromethamine | NSAID | 4.09% |
| Indoprofen | NSAID | 4.23% |
| Tiaprofenic Acid | NSAID | 4.32% |
| Flurbiprofen | NSAID | 5.78% |
| Ketoprofen | NSAID | 6.50% |
| Tolmetin | NSAID | 8.64% |
| Suprofen | NSAID | 8.74% |
| Carprofen | NSAID | 12.2% |
Mechanism of Action: A COX-Independent Pathway
Follow-up studies confirmed that NSAIDs like ibuprofen, naproxen, and ketorolac inhibit caspase catalytic activity at physiologic concentrations both in vitro and in vivo [89]. This inhibition is COX-independent, as demonstrated in studies using COX-deficient cells and a C. elegans model [89].
The mechanism involves direct binding to the catalytic site of caspases, thereby reducing cell death and the generation of pro-inflammatory cytokines like IL-1β under inflammatory conditions [89]. This represents a significant expansion of the known anti-inflammatory mechanism of NSAIDs.
Furthermore, a related class of drugs, NO-releasing NSAIDs (NO-NSAIDs), have also been shown to be potent caspase inhibitors. Their mechanism is postulated to involve the S-nitrosation of the catalytic cysteine residue in caspases like caspase-1, leading to its inactivation [90].
The Research Toolkit: Caspase Inhibitors in Practice
Research Reagent Solutions
The following table lists key reagents essential for conducting research in caspase biology and inhibition.
Table 3: Essential Research Reagents for Caspase Inhibition Studies
| Reagent / Tool | Function / Description | Example Application |
|---|---|---|
| Z-VAD-FMK | Cell-permeable, irreversible pan-caspase inhibitor. Potently inhibits caspase-1 to -10. | Used to broadly inhibit caspase activity to study overall contribution of caspases to a process (e.g., [21] [15]). |
| Ac-YVAD-CHO | Peptide-based, reversible inhibitor with selectivity for caspase-1. | Useful for specifically interrogating the role of inflammatory caspases and inflammasome activity. |
| Ac-DEVD-CHO | Peptide-based, reversible inhibitor with selectivity for executioner caspases like caspase-3. | Used to study the role of apoptotic executioners specifically. |
| Q-VD-OPh | A broad-spectrum caspase inhibitor with enhanced efficacy, permeability, and reduced toxicity in vivo. | Preferred for in vivo studies due to its better safety profile [9]. |
| Fluorogenic Caspase Substrates (e.g., Ac-DEVD-AFC) | Peptides linked to a fluorophore (e.g., AFC). Caspase cleavage releases the fluorophore, generating a measurable signal. | Quantifying caspase enzymatic activity in cell lysates or with recombinant enzymes [89]. |
| Prestwick Chemical Library | A library of 1,280 FDA-approved, off-patent drugs. | Ideal for drug repurposing screens to identify unexpected pharmacological activities, as with NSAIDs [89]. |
Experimental Protocol: Validating Caspase Inhibition In Vitro
This protocol outlines the key steps for testing the caspase inhibitory activity of a compound of interest (e.g., an NSAID) in vitro, based on methodologies from the search results [89] [41].
Title: In Vitro Assessment of Small Molecule Caspase Inhibition
Objective: To determine the potency (IC₅₀) of a test compound against a specific recombinant caspase enzyme.
Materials:
- Recombinant caspase enzyme (e.g., human caspase-4, -1, or -3).
- Fluorogenic caspase substrate (e.g., Ac-DEVD-AFC for caspase-3/7, specific substrates for other caspases).
- Assay buffer (e.g., containing DTT to maintain reducing conditions).
- Black, clear-bottom 96-well microplate.
- Microplate reader capable of kinetic fluorescence measurements.
- Test compounds (e.g., NSAIDs like ibuprofen, naproxen) dissolved in DMSO.
- Positive control inhibitor (e.g., Z-VAD-FMK for pan-caspase inhibition).
Procedure:
- Prepare Reaction Mixture: In a final assay volume of 100 μL per well, add assay buffer, recombinant caspase, and the test compound at a range of concentrations (e.g., 1 nM to 100 μM). Include a vehicle control (DMSO) and a positive inhibition control.
- Pre-incubate: Allow the caspase and inhibitor to incubate for 15-30 minutes at room temperature.
- Initiate Reaction: Add the fluorogenic substrate to start the enzymatic reaction.
- Measure Kinetics: Immediately transfer the plate to the microplate reader and measure the fluorescence (e.g., excitation ~400 nm, emission ~505 nm for AFC) every minute for 60-120 minutes.
- Data Analysis:
- Calculate the initial velocity (V₀) of the reaction for each well from the linear range of the fluorescence-vs-time curve.
- Normalize V₀ values as a percentage of the vehicle control activity.
- Plot the % activity vs. log₁₀ of the inhibitor concentration and fit the data with a sigmoidal dose-response curve to determine the IC₅₀ value.
The workflow for this screening and validation process is summarized below.
Clinical Implications and Future Directions
The discovery of NSAIDs as caspase inhibitors has significant clinical implications. It expands the understanding of their anti-inflammatory mechanism, which may contribute to their efficacy and also explain some of their adverse effects, particularly related to the blockade of beneficial apoptosis [89]. This knowledge opens avenues for drug repurposing and the design of next-generation anti-inflammatory drugs with improved safety profiles [89] [90].
This finding must also be contextualized within the broader challenges of developing therapeutic caspase inhibitors. While natural and synthetic caspase inhibitors have shown promise, most have faced challenges in clinical trials due to inadequate efficacy, poor target specificity, or adverse side effects [9]. The chart below classifies the main types of caspase inhibitors.
The unexpected crossover of NSAIDs into caspase inhibition underscores the complexity of drug mechanisms and offers a promising pathway for rapid clinical translation. Future work will focus on delineating the specific structural interactions, optimizing selectivity, and exploring their potential in treating caspase-driven pathologies beyond inflammation.
Caspases, an evolutionarily conserved family of cysteine-dependent proteases, are master regulators of vital cellular processes including apoptosis, proliferation, differentiation, and inflammatory responses [9] [52]. Dysregulation of caspase-mediated pathways constitutes a fundamental mechanism in the pathogenesis of various diseases, rendering caspases attractive therapeutic targets for conditions ranging from inflammatory and neurological disorders to cancer [9] [91]. The development of caspase inhibitors has thus emerged as a promising therapeutic strategy.
Among these inhibitors, Z-VAD-FMK (benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone) stands as a prototypical, irreversible pan-caspase inhibitor widely utilized in basic research [23]. However, its position within the broader therapeutic landscape of caspase inhibition is distinct. This application note delineates the specific role of Z-VAD-FMK as a foundational research tool, contrasting it with clinical-stage caspase inhibitors, and provides detailed experimental protocols for its application in mechanistic and therapeutic studies.
The Caspase Inhibitor Landscape: From Research Tools to Clinical Candidates
The journey of caspase inhibitors from bench to bedside has been challenging. While numerous inhibitors have been designed, only a handful have progressed to clinical trials, and none have yet achieved approval for routine clinical use [9]. The high homology between caspase family members, coupled with an incomplete understanding of their non-apoptotic functions and the consistent challenges of inadequate efficacy, poor specificity, and adverse side effects, has hindered clinical translation [9] [91].
Table 1: Classification and Characteristics of Prominent Caspase Inhibitors
| Inhibitor Name | Primary Target(s) | Chemical Class | Development Stage | Key Characteristics & Findings |
|---|---|---|---|---|
| Z-VAD-FMK | Pan-caspase (broad-spectrum) | Peptide-based, FMK-derivative | Research Tool Compound | Irreversible inhibitor; cell-permeable; used extensively in vitro and in vivo to study cell death mechanisms [23]. |
| VX-740 (Pralnacasan) | Caspase-1 | Peptidomimetic | Clinical Trials (Terminated) | Showed significant potency for rheumatoid arthritis and osteoarthritis in trials; development halted due to liver toxicity in animal models [9]. |
| VX-765 (Belnacasan) | Caspase-1 | Peptidomimetic | Clinical Trials (Terminated) | More potent than VX-740 for inflammatory diseases; trials terminated due to liver toxicity concerns [9]. |
| IDN-6556 (Emricasan) | Pan-caspase | Peptidomimetic | Clinical Trials (Terminated) | Investigated for liver diseases; preclinical and clinical studies showed efficacy, but development was terminated due to side effects from extended treatment [9]. |
| Q-VD-OPh | Pan-caspase | Peptide-based | Advanced Preclinical Research | Broad-spectrum inhibitor with enhanced efficacy, permeability, and reduced toxicity in vivo even at high concentrations [9]. |
Z-VAD-FMK occupies a critical niche in this landscape. It functions as an irreversible, broad-spectrum caspase inhibitor that binds covalently to the catalytic cysteine residue in the active site of most caspases [73]. Its core structure consists of a peptide recognition sequence (Val-Ala-Asp) linked to a fluoromethyl ketone (FMK) electrophilic warhead. The FMK group reacts with the thiol group of the catalytic cysteine, forming a thiomethyl ketone adduct that permanently inactivates the enzyme [73]. The FMK group also improves cell permeability and reduces cellular toxicity compared to chloromethyl or bromomethyl ketones [9].
Table 2: Comparative Analysis of Z-VAD-FMK and Clinical-Stage Inhibitors
| Feature | Z-VAD-FMK | Clinical-Stage Peptidomimetics (e.g., VX-765, IDN-6556) |
|---|---|---|
| Primary Application | Fundamental research, proof-of-concept studies, in vitro and in vivo model validation | Targeted therapeutic intervention for specific human diseases |
| Specificity | Low (pan-caspase inhibition); can inhibit other proteases | Higher (designed for specific caspases, e.g., caspase-1) |
| Toxicity Profile | Can promote necroptosis under certain conditions; used with caution in complex models [7] | Terminated due to organ toxicity (e.g., liver) or other undisclosed adverse effects [9] |
| Regulatory Status | Not for human use; sold as a research reagent | Have undergone Phase I/II clinical trials in humans |
| Key Utility | Essential for establishing causal roles of caspases in biological processes | Provide insights into the challenges of clinical caspase inhibition |
Application Notes and Experimental Protocols
Protocol 1: Utilizing Z-VAD-FMK to Attenuate Endotoxic ShockIn Vivo
This protocol is adapted from a study demonstrating that Z-VAD-FMK alleviates lipopolysaccharide (LPS)-induced endotoxic shock in mice, highlighting its anti-inflammatory potential [7].
Objective: To investigate the protective effect of pan-caspase inhibition against LPS-induced systemic inflammation and mortality.
Materials:
- Research Reagent Solutions:
- Z-VAD-FMK: Reconstitute in DMSO or saline to a stock concentration of 10-20 mg/mL. Aliquots and store at -20°C.
- Lipopolysaccharide (LPS): Prepare in sterile saline.
- Experimental Animals: Female C57BL/6 mice, 6-8 weeks old.
- Vehicle Control: Saline or equivalent volume of DMSO in saline.
Methodology:
- Animal Pre-treatment: Administer Z-VAD-FMK (dose range: 5-20 µg per gram of body weight) or vehicle control via intraperitoneal (i.p.) injection 2 hours prior to LPS challenge [7].
- Disease Induction: Induce endotoxic shock by i.p. injection of a high dose of LPS (e.g., 25-50 µg/g body weight for survival studies; 10 µg/g for cytokine/organ damage analysis) [7].
- Post-treatment (Optional): To model therapeutic intervention, Z-VAD-FMK can be administered after LPS challenge [7].
- Monitoring and Analysis:
- Survival: Monitor and record mortality every hour for the duration of the experiment.
- Serum Collection: At 6 hours post-LPS challenge, collect blood and isolate serum for cytokine analysis (e.g., TNF-α, IL-6) via ELISA.
- Tissue Harvest: At 12 hours post-LPS, harvest organs (liver, lung, spleen) for histopathological examination (H&E staining) and analysis of immune cell populations by flow cytometry [7].
Key Findings and Interpretation:
- Treatment with Z-VAD-FMK significantly reduces mortality and alleviates tissue pathology in the liver and lung [7].
- The protective mechanism is twofold: Z-VAD-FMK promotes nitric oxide-mediated necroptosis of macrophages, reducing their numbers, and simultaneously inhibits pro-inflammatory cytokine secretion from surviving macrophages [7].
- This protocol underscores a critical consideration: Z-VAD-FMK does not universally block cell death but can shift the mode of death from apoptosis to necroptosis, a process that must be carefully interpreted in experimental models [7].
Protocol 2: Enhancing Graft Survival in Ovarian Tissue Transplantation
This protocol details the use of Z-VAD-FMK to mitigate ischemia/reperfusion injury and improve the survival of human ovarian tissue after transplantation, a key application in fertility preservation [72].
Objective: To reduce caspase-mediated apoptosis in cryopreserved-thawed human ovarian fragments following xenotransplantation.
Materials:
- Research Reagent Solutions:
- Z-VAD-FMK Stock: Prepare a 50 mM stock solution in DMSO.
- Human Ovarian Tissue: Cryopreserved cortical strips.
- Collagen Matrix: Type I collagen gel for tissue encapsulation.
- SCID Mice: 8-week-old female mice as transplantation hosts.
Methodology:
- Thawing and Preparation: Thaw cryopreserved human ovarian fragments using standard protocols [72].
- Experimental Group Setup:
- Control Group: Embed thawed ovarian fragments in a collagen matrix.
- Z-VAD-FMK Group: Embed fragments in a collagen matrix supplemented with 50 µM Z-VAD-FMK [72].
- Transplantation: Surgically transplant the encapsulated ovarian fragments under the ovarian bursa of SCID mice.
- Graft Recovery and Analysis: Recover grafts at two critical time points:
- Early (3 days post-grafting): Assess initial apoptosis wave and tissue integrity.
- Late (3 weeks post-grafting): Evaluate long-term follicular survival and function.
- Assessment:
- Follicular Density: Count morphologically intact primordial and primary follicles per mm² of tissue.
- Apoptosis Measurement: Analyze tissue sections by TUNEL assay or immunohistochemistry for cleaved caspase-3.
- Western Blotting: Detect apoptosis markers like cleaved PARP in tissue lysates [72].
Key Findings and Interpretation:
- Z-VAD-FMK treatment significantly improves the density of primary follicles after 3 weeks of transplantation but may not show a significant effect at the earlier 3-day time point [72].
- This indicates that Z-VAD-FMK primarily protects against secondary, caspase-dependent apoptotic pathways during the later phases of graft integration rather than the immediate ischemic insult.
- The application of Z-VAD-FMK via a collagen scaffold provides a effective method for localized, sustained delivery of the inhibitor to the graft site, minimizing potential systemic effects.
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Reagents for Caspase Inhibition Studies with Z-VAD-FMK
| Reagent / Material | Function / Role in Experiment | Example Application / Note |
|---|---|---|
| Z-VAD-FMK | Irreversible, broad-spectrum caspase inhibitor. Core investigative tool. | Used in a wide range of models (in vitro, ex vivo, in vivo) to implicate caspases in a process. [7] [72] |
| Vehicle Control (DMSO/Saline) | Control for the solvent used to dissolve Z-VAD-FMK. | Critical for attributing observed effects to the inhibitor and not the solvent. |
| Lipopolysaccharide (LPS) | Potent inducer of inflammation and caspase activation (e.g., via caspase-11). | Used to model inflammatory shock and sepsis. [7] |
| Cryopreserved Human Tissues | Ex vivo model for studying ischemia/reperfusion injury. | Ovarian tissue fragments used in fertility preservation research. [72] |
| Collagen Matrix (Type I) | 3D scaffold for tissue encapsulation and localized drug delivery. | Provides sustained release of Z-VAD-FMK to the graft site. [72] |
| Fluorogenic Caspase Substrates (e.g., Ac-DEVD-AFC) | Detect and quantify caspase enzyme activity in cell or tissue lysates. | Substrate cleavage releases a fluorescent signal proportional to caspase activity. |
| Antibodies (Cleaved Caspase-3, PARP) | Detect caspase activation and apoptotic signaling in tissues via IHC/Western Blot. | Provides spatial information and confirmation of pathway inhibition. [72] |
Z-VAD-FMK remains an indispensable tool for deconvoluting the complex roles of caspases in cellular processes and disease models. Its utility in establishing proof-of-concept, as demonstrated in protocols for endotoxic shock and tissue transplantation, provides the foundational evidence that can guide the development of more selective, clinically viable caspase inhibitors. While clinical-stage inhibitors have faced significant hurdles, the research facilitated by Z-VAD-FMK continues to refine our understanding of caspase biology, paving the way for future therapeutic strategies that more precisely modulate specific caspases or their downstream effectors to achieve clinical success.
Conclusion
Z-VAD-FMK remains a cornerstone tool for dissecting caspase-dependent processes in cell death and inflammation, with demonstrated efficacy across numerous preclinical disease models. Its irreversible pan-caspase inhibition provides broad research utility but also presents challenges in complex biological systems where pathway crosstalk can lead to paradoxical effects. Future directions should focus on developing more specific caspase inhibitors with improved safety profiles, understanding the non-apoptotic roles of caspases that may be affected by inhibition, and translating the protective effects observed in animal models to clinical applications. The continued investigation of caspase inhibition holds significant promise for therapeutic interventions in inflammatory, degenerative, and ischemic conditions, with Z-VAD-FMK serving as a critical foundation for these advances.
References
- 1. Caspases as master regulators of programmed cell death [https://pmc.ncbi.nlm.nih.gov/articles/PMC12229594/]
- 2. structural and molecular mechanisms and functions in cell ... [https://www.nature.com/articles/s41421-025-00791-3]
- 3. A long way to go: caspase inhibitors in clinical use - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC8519909/]
- 4. Caspase family in autoimmune diseases [https://www.sciencedirect.com/science/article/abs/pii/S1568997224002052]
- 5. Caspases: structural and molecular mechanisms and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12052993/]
- 6. Caspase-8 in inflammatory diseases: a potential therapeutic ... [https://cmbl.biomedcentral.com/articles/10.1186/s11658-024-00646-x]
- 7. The Caspase Inhibitor Z-VAD-FMK Alleviates Endotoxic Shock ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6687755/]
- 8. Caspases as Targets for Drug Development - NCBI - NIH [https://www.ncbi.nlm.nih.gov/books/NBK6578/]
- 9. A long way to go: caspase inhibitors in clinical use [https://www.nature.com/articles/s41419-021-04240-3]
- 10. The history of Z-VAD-FMK, a tool for understanding ... [https://www.sciencedirect.com/science/article/abs/pii/S0065128104700719]
- 11. Z-VAD-FMK | CAS 187389-52-2 | Pan-specific caspase ... [https://www.stressmarq.com/products/small-molecules/inhibitor/z-vad-fmk-sih-557/?srsltid=AfmBOoqNOeHq1IwllVbqp1pFjwUC9dzrFi1Qh_maeeVlI3pC8-Fq9veq]
- 12. Z-VAD-FMK: Irreversible Pan-Caspase Inhibitor for Apoptos... [https://www.dihydro-b-erythroidine.com/index.php?g=Wap&m=Article&a=detail&id=19]
- 13. Kinetic and structural characterization of caspase-3 and ... [https://pubmed.ncbi.nlm.nih.gov/20580860/]
- 14. Z-VAD(OMe)-FMK, Cell permeable, irreversible pan ... [https://www.abcam.com/en-us/products/biochemicals/z-vadome-fmk-cell-permeable-irreversible-pan-caspase-inhibitor-ab120487]
- 15. The Caspase Inhibitor Z-VAD-FMK Alleviates Endotoxic ... [https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2019.01824/full]
- 16. Caspase inhibitors promote alternative cell death pathways [https://pubmed.ncbi.nlm.nih.gov/17062895/]
- 17. Cellular autophagy, an unbidden effect of caspase by... inhibition [https://pmc.ncbi.nlm.nih.gov/articles/PMC9177521/]
- 18. JPP No 4/2015 article 01 [https://jpp.krakow.pl/journal/archive/08_15/articles/01_article.html]
- 19. Live Cell Imaging of Caspase Activation for High Content ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3613133/]
- 20. zVAD-fmk upregulates caspase-9 cleavage and activity in ... [https://www.sciencedirect.com/science/article/pii/S0167488912001292]
- 21. Pan-caspase inhibitor protects against noise-induced ... [https://www.frontiersin.org/journals/neuroscience/articles/10.3389/fnins.2025.1497773/full]
- 22. Caspase inhibitors: viral, cellular and chemical [https://www.nature.com/articles/4402034]
- 23. Z-VAD-FMK - an overview [https://www.sciencedirect.com/topics/chemistry/z-vad-fmk]
- 24. An Allosteric Circuit in Caspase-1 - PMC - PubMed Central [https://pmc.ncbi.nlm.nih.gov/articles/PMC2626611/]
- 25. Structural Basis for the Inhibition of Caspase-3 by XIAP [https://www.sciencedirect.com/science/article/pii/S0092867401002744]
- 26. Identification of Allosteric Inhibitors against Active Caspase-6 [https://www.nature.com/articles/s41598-019-41930-7]
- 27. Caspase-9 Activation of Procaspase-3 but not ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8489496/]
- 28. Conformational transitions of caspase-6 in substrate ... [https://www.sciencedirect.com/science/article/pii/S200103702100307X]
- 29. zVAD-fmk upregulates caspase-9 cleavage and activity in ... [https://pubmed.ncbi.nlm.nih.gov/22613767/]
- 30. Caspase Inhibitor zVAD-fmk Protects Against Acute ... [https://pubmed.ncbi.nlm.nih.gov/27324074/]
- 31. Z-VAD-FMK - Pan-Caspase inhibitor [https://www.invivogen.com/z-vad-fmk]
- 32. Z-VAD(OMe)-FMK #60332 [https://www.cellsignal.com/products/activators-inhibitors/z-vad-ome-fmk/60332?srsltid=AfmBOooa8rJIaXl7Jj6zB9WZtS3fZe3lpyzd4hjzWzbjb9TmmbqwIyB9]
- 33. Z-VAD-FMK: Strategic Caspase Inhibition for Translational... [https://z-fa-fmk.com/index.php?g=Wap&m=Article&a=detail&id=6]
- 34. The Quantitative-Phase Dynamics of Apoptosis and Lytic ... [https://www.nature.com/articles/s41598-020-58474-w]
- 35. A comparative study of apoptosis, pyroptosis, necroptosis, and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10898734/]
- 36. Cellular autophagy, an unbidden effect of caspase inhibition ... [https://pubmed.ncbi.nlm.nih.gov/35043564/]
- 37. Caspase Inhibitor Z-VAD-FMK [https://www.promega.com/products/cell-health-assays/apoptosis-assays/caspase-inhibitor-z_vad_fmk/]
- 38. Structure of human caspase-6 in complex with Z-VAD-FMK [https://pubmed.ncbi.nlm.nih.gov/21820899/]
- 39. Z-VAD(OMe)-FMK #60332 [https://www.cellsignal.com/products/activators-inhibitors/z-vad-ome-fmk/60332?srsltid=AfmBOoq3BOW445qdhqmlhdqBtwCJrqFDzwV_3eWbUqA_LLO9YdjfvBJr]
- 40. Caspase inhibitor Z-VAD-FMK enhances the freeze-thaw ... [https://www.stemcell.com/caspase-inhibitor-z-vad-fmk-enhances-the-freeze-thaw-survival-rate-of-human-embryonic-stem-cells.html]
- 41. In vitro evaluation of the anti-apoptotic drug Z-VAD-FMK on ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4615917/]
- 42. MEROPS - the Peptidase Database [https://www.ebi.ac.uk/merops/cgi-bin/smi_summary?mid=J00084]
- 43. Z-VAD(OMe)-FMK #60332 [https://www.cellsignal.com/products/activators-inhibitors/z-vad-ome-fmk/60332?srsltid=AfmBOootbJIts3zWHlfF4L_LDxn455ckABcX_2NJY1M53P8uGWHId3dL]
- 44. Norepinephrine exacerbates LPS-induced cardiomyopathy ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12344832/]
- 45. Z-VAD-FMK, General Caspase Inhibitor [https://www.bdbiosciences.com/en-us/products/reagents/flow-cytometry-reagents/research-reagents/single-color-antibodies-ruo/z-vad-fmk-general-caspase-inhibitor.550377]
- 46. Z-VAD(OMe)-FMK (Synonyms: Z-Val-Ala-Asp(OMe) [https://www.medchemexpress.com/Z-VAD-FMK.html?srsltid=AfmBOopNnD6xIjQCM7LtOHydn5Mr7yFtqyKzxl1Y--iAbAchsTwjm1B9]
- 47. Cell death signaling and immune regulation [https://cmbl.biomedcentral.com/articles/10.1186/s11658-025-00784-w]
- 48. Caspase inhibitor z-VAD-FMK increases the survival of hair ... [https://www.sciencedirect.com/science/article/abs/pii/S0304394020303591]
- 49. Programmed cell death in atherosclerosis and vascular ... [https://www.nature.com/articles/s41419-022-04923-5]
- 50. z-VAD-fmk-induced non-apoptotic cell death of macrophages [https://pubmed.ncbi.nlm.nih.gov/16874073/]
- 51. Caspase Substrates and Inhibitors - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC3721276/]
- 52. Caspases as master regulators of programmed cell death [https://www.nature.com/articles/s12276-025-01470-9]
- 53. Pharmacological inhibition of apoptosis, necroptosis, and ... [https://www.nature.com/articles/s41598-025-17275-9]
- 54. Therapeutic Potential of Emricasan, a Pan-Caspase ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11988121/]
- 55. Suppression of human T cell proliferation by the caspase ... [https://www.sciencedirect.com/science/article/pii/S0041008X12003985]
- 56. Z-VAD-FMK | pan-Caspase Inhibitor [https://www.selleckchem.com/products/z-vad-fmk-pan-caspase-inhibitor.html]
- 57. Caspase-8 inhibition improves the outcome of bacterial ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10394171/]
- 58. zVAD-induced necroptosis in L929 cells depends on ... [https://www.nature.com/articles/cdd201072]
- 59. Caspase Inhibition Prevents Tumor Necrosis Factor-α ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5222974/]
- 60. Pan-caspase inhibitors induce necroptosis via ROS ... [https://www.sciencedirect.com/science/article/abs/pii/S0014482719302113]
- 61. Apoptosis: A Comprehensive Overview of Signaling ... [https://www.mdpi.com/2073-4409/13/22/1838]
- 62. Z-VAD-FMK [https://www.enzo.com/product/z-vad-fmk/]
- 63. Z-VAD-FMK – Pan-Caspase Inhibitor [https://www.apexbt.com/z-vad-fmk.html]
- 64. Proinflammatory cytokines sensitise mesenchymal stromal ... [https://www.nature.com/articles/s41420-025-02412-0]
- 65. Inhibition of Apoptosis by Z-VAD-fmk in SMN-depleted S2 ... [https://www.sciencedirect.com/science/article/pii/S0021925820841386]
- 66. Z-VAD(OMe)-FMK [https://bpsbioscience.com/z-vadome-fmk-27313]
- 67. Single-Cell and Population-Level Analyses Using Real ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6810872/]
- 68. Robust high-throughput kinetic analysis of apoptosis with ... [https://www.nature.com/articles/cddis2016332]
- 69. Kaempferol mitigates Endoplasmic Reticulum Stress Induced Cell... [https://www.nature.com/articles/s41598-018-20499-7?error=cookies_not_supported&code=430a2db1-cc20-4bc5-b20d-76652dc57d25]
- 70. Caspase Inhibitor Z-VAD-FMK [https://worldwide.promega.com/products/cell-health-assays/apoptosis-assays/caspase-inhibitor-z_vad_fmk/]
- 71. Z-VAD-FMK (Z-VAD(OH)-FMK) | Pan-Caspase Inhibitor [https://www.medchemexpress.com/z-vad-fmk-1.html?srsltid=AfmBOorvqGAzwlS-dJOlNWTK5P8bkNVjVkZ3Wk6vk3L95YJItIQFZ2l9]
- 72. Evaluation of Z-VAD-FMK as an anti-apoptotic drug to prevent ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6420548/]
- 73. Peptidyl Fluoromethyl Ketones and Their Applications in ... [https://www.mdpi.com/1420-3049/25/17/4031]
- 74. z-VAD-fmk augmentation of TNF alpha-stimulated ... [https://pubmed.ncbi.nlm.nih.gov/15572588/]
- 75. Cuproptosis: molecular mechanisms, cancer prognosis, and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11752808/]
- 76. Current understanding of eryptosis [https://www.nature.com/articles/s41419-025-07784-w]
- 77. An activation-based high throughput screen identifies caspase ... [https://pubs.rsc.org/en/content/articlehtml/2025/cb/d5cb00017c]
- 78. Z-VAD-FMK: Advanced Strategies for Apoptosis Inhibition a... [https://www.2-amino-datp.com/index.php?g=Wap&m=Article&a=detail&id=23]
- 79. Caspase-Glo® 3/7 Assay System [https://www.promega.com/products/cell-health-assays/apoptosis-assays/caspase_glo-3_7-assay-systems/]
- 80. Q-VD-OPh, a broad spectrum caspase inhibitor with potent ... [https://link.springer.com/article/10.1023/A:1024116916932]
- 81. Q-VD-OPh, a broad spectrum caspase inhibitor with potent ... [https://pubmed.ncbi.nlm.nih.gov/12815277/]
- 82. Q-VD-OPH | CAS 1135695-98-5 [https://www.scbt.com/p/q-vd-oph-1135695-98-5?srsltid=AfmBOor3eS8EgLlaJVqQ--kIQUw38KU57gZbDUcZGUgWrldq2GOKjBFL]
- 83. Emricasan, a pan caspase inhibitor, improves survival and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6347405/]
- 84. Therapeutic Potential of Emricasan, a Pan-Caspase ... [https://www.mdpi.com/2073-4409/14/7/498]
- 85. Emricasan: Uses, Interactions, Mechanism of Action [https://go.drugbank.com/drugs/DB05408]
- 86. Suppression of human T cell proliferation by the caspase ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3875211/]
- 87. From mechanism to application: programmed cell death ... [https://www.sciencedirect.com/science/article/pii/S2452199X25002889]
- 88. Caspase Inhibitors: Prospective Therapies for Stroke - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC9214550/]
- 89. NSAIDs are Caspase Inhibitors - PMC - PubMed Central [https://pmc.ncbi.nlm.nih.gov/articles/PMC5357154/]
- 90. NO-releasing NSAIDs are caspase inhibitors [https://www.sciencedirect.com/science/article/abs/pii/S1471490601019044]
- 91. The multifunctionality of the caspase family of proteases [https://www.sciencedirect.com/science/article/abs/pii/S0098299725000755]